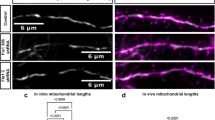
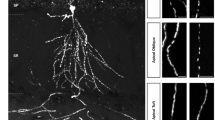
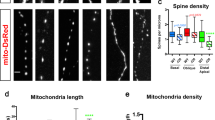
Abstract
Neuronal mitochondria display distinct morphologies across compartments, with dendritic mitochondria being elongated and axonal ones shorter, and their morphologies are dynamically changed via fusion and fission machineries. Mitochondrial structural abnormalities are common in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, yet systematic evaluation of therapeutic targets remains limited. Here, we tested key mitochondrial shape regulators, mitofusin 1/2 for fusion and Mff/Fis1 for fission, in an α-synucleinopathy model. Using MitoVis, a deep learning-based neuronal mitochondrial image analysis tool, we achieved rapid, compartment-specific analysis of mitochondrial morphologies. Among all interventions, Fis1 knockdown most effectively protected mitochondrial structure to control levels without inducing over-elongation of axonal mitochondria, which was linked to abnormal Ca2+ dynamics. While all manipulations preserved dendritic spine loss, Fis1 optimally maintained axonal mitochondrial function. These findings demonstrate a high-throughput screening approach for mitochondrial regulators and highlight Fis1 as a promising preventive/therapeutic target. Our results support targeting mitochondrial morphology as a viable strategy for treating α-synucleinopathy and potentially other mitochondria-related neurodegenerative diseases.
Similar content being viewed by others
Data availability
All data reported in this paper will be shared by the corresponding authors upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this work paper is available from the corresponding authors upon request.
References
Rangaraju, V. et al. Pleiotropic mitochondria: the influence of mitochondria on neuronal development and disease. J. Neurosci. 39, 8200–8208 (2019).
Lee, A., Hirabayashi, Y., Kwon, S. K., Lewis, T. L. Jr. & Polleux, F. Emerging roles of mitochondria in synaptic transmission and neurodegeneration. Curr. Opin. Physiol. 3, 82–93 (2018).
Divakaruni, S. S. et al. Long-term potentiation requires a rapid burst of dendritic mitochondrial fission during induction. Neuron 100, 860–875.e867 (2018).
Rangaraju, V., Lauterbach, M. & Schuman, E. M. Spatially stable mitochondrial compartments fuel local translation during plasticity. Cell 176, 73–84.e15 (2019).
Ashrafi, G., de Juan-Sanz, J., Farrell, R. J. & Ryan, T. A. Molecular tuning of the axonal mitochondrial Ca(2+) uniporter ensures metabolic flexibility of neurotransmission. Neuron 105, 678–687.e675 (2020).
Kwon, S. K. et al. LKB1 regulates mitochondria-dependent presynaptic calcium clearance and neurotransmitter release properties at excitatory synapses along cortical axons. PLoS Biol. 14, e1002516 (2016).
Lewis, T. L. Jr, Kwon, S.-K., Lee, A., Shaw, R. & Polleux, F. MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat. Commun. 9, 5008 (2018).
Rangaraju, V., Calloway, N. & Ryan, T. A. Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014).
Sun, T., Qiao, H., Pan, P.-Y., Chen, Y. & Sheng, Z.-H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4, 413–419 (2013).
Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct. Target. Ther. 8, 333 (2023).
Giorgi, C., Marchi, S. & Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 19, 713–730 (2018).
Geibl, F. F. et al. α-Synuclein pathology disrupts mitochondrial function in dopaminergic and cholinergic neurons at-risk in Parkinson’s disease. Mol. Neurodegeneration 19, 69 (2024).
Zhang, L. et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci. Rep. 6, 18725 (2016).
Lee, A. et al. Aβ42 oligomers trigger synaptic loss through CAMKK2-AMPK-dependent effectors coordinating mitochondrial fission and mitophagy. Nat. Commun. 13, 4444 (2022).
Ganjam, G. K. et al. Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis. 10, 865 (2019).
Nakamura, K. et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 286, 20710–20726 (2011).
Vicario, M., Cieri, D., Brini, M. & Calì, T. The close encounter between alpha-synuclein and mitochondria. Front. Neurosci. 12, 388 (2018).
Zambon, F. et al. Cellular α-synuclein pathology is associated with bioenergetic dysfunction in Parkinson’s iPSC-derived dopamine neurons. Hum. Mol. Genet. 28, 2001–2013 (2019).
Volpicelli-Daley, L. A. et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011).
Volpicelli-Daley, L. A., Luk, K. C. & Lee, V. M. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014).
Wu, Q. et al. α-Synuclein (αSyn) preformed fibrils induce endogenous αSyn aggregation, compromise synaptic activity and enhance synapse loss in cultured excitatory hippocampal neurons. J. Neurosci. 39, 5080–5094 (2019).
Choi, J. et al. MitoVis: a unified visual analytics system for end-to-end neuronal mitochondria analysis. IEEE Trans. Vis. Comput. Graph. 30, 3457–3473 (2024).
Tábara, L.-C., Segawa, M. & Prudent, J. Molecular mechanisms of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 26, 123–146 (2025).
Wang, X. et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 29, 9090–9103 (2009).
Wang, X. et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 21, 1931–1944 (2012).
Cheng, W. C. et al. Fis1 deficiency selects for compensatory mutations responsible for cell death and growth control defects. Cell Death Differ. 15, 1838–1846 (2008).
Fannjiang, Y. et al. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 18, 2785–2797 (2004).
Liu, X., Li, T., Tu, X., Xu, M. & Wang, J. Mitochondrial fission and fusion in neurodegenerative diseases:Ca(2+) signalling. Mol. Cell Neurosci. 132, 103992 (2025).
Jang, D. C. et al. Asymmetric distribution of mitochondrial Ca2+ regulators specifies compartment-specific mitochondrial function and neuronal development. Preprint at https://www.biorxiv.org/content/10.1101/2025.06.23.660978v1 (2025).
de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008).
Zahedi, A. et al. Deep analysis of mitochondria and cell health using machine learning. Sci. Rep. 8, 16354 (2018).
Rohani, A., Kashatus, J. A., Sessions, D. T., Sharmin, S. & Kashatus, D. F. Mito Hacker: a set of tools to enable high-throughput analysis of mitochondrial network morphology. Sci. Rep. 10, 18941 (2020).
Fischer, C. A. et al. MitoSegNet: easy-to-use deep learning segmentation for analyzing mitochondrial morphology. iScience 23, 101601 (2020).
Vojtová, J. et al. A fully automated morphological analysis of yeast mitochondria from wide-field fluorescence images. Sci. Rep. 14, 30144 (2024).
Varkuti, B. H. et al. Neuron-based high-content assay and screen for CNS active mitotherapeutics. Sci. Adv. 6, eaaw8702 (2020).
Kamp, F. et al. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 29, 3571–3589 (2010).
Krzystek, T. J. et al. Differential mitochondrial roles for α-synuclein in DRP1-dependent fission and PINK1/Parkin-mediated oxidation. Cell Death Dis. 12, 796 (2021).
Furlong, R. M., O’Keeffe, G. W., O’Neill, C. & Sullivan, A. M. Alterations in α-synuclein and PINK1 expression reduce neurite length and induce mitochondrial fission and Golgi fragmentation in midbrain neurons. Neurosci. Lett. 720, 134777 (2020).
Grassi, D. et al. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 115, E2634–e2643 (2018).
Butler, E. K. et al. The mitochondrial chaperone protein TRAP1 mitigates α-Synuclein toxicity. PLoS Genet. 8, e1002488 (2012).
Xie, W. & Chung, K. K. Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J. Neurochem. 122, 404–414 (2012).
Ordonez, D. G., Lee, M. K. & Feany, M. B. α-Synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97, 108–124.e106 (2018).
Portz, P. & Lee, M. K. Changes in Drp1 function and mitochondrial morphology are associated with the α-synuclein pathology in a transgenic mouse model of Parkinson’s disease. Cells 10, 885 (2021).
Bapat, O. et al. VAP spatially stabilizes dendritic mitochondria to locally support synaptic plasticity. Nat. Commun. 15, 205 (2024).
Filichia, E., Hoffer, B., Qi, X. & Luo, Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci. Rep. 6, 32656 (2016).
Qi, X., Qvit, N., Su, Y.-C. & Mochly-Rosen, D. A. novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 126, 789–802 (2013).
Su, Y.-C. & Qi, X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum. Mol. Genet. 22, 4545–4561 (2013).
Bido, S., Soria, F. N., Fan, R. Z., Bezard, E. & Tieu, K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-α-synuclein rat model of Parkinson’s disease. Sci. Rep. 7, 7495 (2017).
Rinaldi, C. & Wood, M. J. A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 14, 9–21 (2018).
Chen, H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 (2003).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Acknowledgements
We also thank all members of the Kwon lab and collaborators for feedback and discussion along the way. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019M3E5D2A0106379412, 2020R1C1C1006386, RS-2022-NR067817, RS-2023-00264980 to S.-K.K.; RS-2021-NR061738 to D.C.J.) and KIST Program (26Z9001 and 26E0131 to S.-K.K.).
Author information
Authors and Affiliations
Contributions
S.Y.K., K.H., S-K.K. conceptualized the study. S.Y.K., J.Y.C., D.C.J., P.L., G.S.H., J.K., W-K.J., K.H., S-K.K. performed the study and S.Y.K., J.Y.C, D.C.J., P.L. analyzed data with a manual way or a software. S.Y.K. and S-K.K. originally wrote the paper, and all authors reviewed and edited it.
Corresponding author
Ethics declarations
Competing interests
S.Y.K. and S-K.K. are preparing the patent application related to the contents of this article, in which S.Y.K. and S-K.K. are listed as inventors. J.Y.C. and W-K.J. are co-founders of VIENCE Inc. Other authors declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, S.Y., Choi, J., Jang, D.C. et al. Systematic evaluation of mitochondrial morphology regulators for amelioration of neuronal α-synucleinopathy. npj Parkinsons Dis. (2026). https://doi.org/10.1038/s41531-026-01277-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-026-01277-z