Abstract
We aimed to examine the association between gut permeability biomarkers (LBP, sCD14) and insulin resistance (IR) in schizophrenia. In this cross-sectional study of 91 patients, IR (HOMA-IR ≥ 3.0) was associated with higher LBP, C-reactive protein, body mass index (BMI), and lower physical activity. Logistic regression identified LBP and BMI as independent risk factors, whereas physical activity was protective. These findings suggest altered gut permeability could influence glucose metabolism dysfunction in schizophrenia.
Similar content being viewed by others
Metabolic comorbidities in schizophrenia pose a significant public health concern, reducing life quality and expectancy1. Glucose metabolism impairments are prevalent in these patients2, though the underlying causes remain unclear. Although antipsychotics contribute to metabolic alterations3, glucose dysregulation is also found in drug-naive first-episode cases2, suggesting other factors like genetics and gut-brain axis disruptions4.
Gut-brain axis disturbances are found in several mental illnesses5, including altered microbiota composition and metabolites, and gut permeability6. Increased permeability facilitates translocation of lipopolysaccharides from Gram-negative bacteria into systemic circulation to bind lipopolysaccharide-binding protein (LBP), recognized by immune cells through soluble cluster of differentiation 14 (sCD14), triggering inflammation7. LBP, with long half-life and endotoxin exposure link, is a reliable biomarker of gut permeability, whereas sCD14 reflects downstream immune activation8. Both are elevated in patients with schizophrenia compared with healthy controls9; however, the role of increased gut permeability in glucose metabolism dysregulation in schizophrenia remains unclear despite independent associations with this disorder10 and insulin resistance (IR)11.
We hypothesized that IR in schizophrenia may be influenced by altered gut permeability and therefore: (1) described patient profiles (sociodemographic, clinical, lifestyle, LBP/sCD14) by IR status; (2) examined associations between these biomarkers and clinical variables; and (3) built a model to identify factors, especially gut permeability, linked to IR.
Our sample included 91 patients with schizophrenia (mean age=40.9 ± 12.3, 40.7% women), with IR estimated through the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)4, using a ≥ 3.0 threshold.
Fifty-one patients had IR, while 40 did not. Individuals with HOMA-IR ≥ 3.0 had higher LBP (U = 554.0, p < 0.001) and C-reactive protein (CRP) (U = 615.0, p = 0.001) levels, higher body mass index (BMI) (t = −5.640, p < 0.001) and a lower prevalence of vigorous/moderate physical activity assessed with the International Physical Activity Questionnaire (IPAQ)12 (X2 = 6.873, p = 0.009). We found no differences in other demographic and clinical variables, including toxic habits and adherence to Mediterranean diet (Table 1).
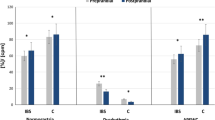
There was positive association between HOMA-IR and LBP (rho = 0.357, p < 0.001), CRP (rho = 0.320, p = 0.002) and BMI (rho = 0.614, p < 0.001). Age, years of evolution, chlorpromazine equivalent doses (CPZ-ED) and clinical severity by CGI-SCH were not correlated (Fig. 1).
* p < 0.05, ** p < 0.01, *** p < 0.001. BMI body mass index, CGI-SCH Clinical Global Impression-Schizophrenia, CPZ-ED chlorpromazine equivalent doses for antipsychotics, CRP C-reactive protein, HOMA-IR Homeostasis Model Assessment of Insulin Resistance, LBP lipopolysaccharide-binding protein, sCD14 soluble cluster of differentiation 14.
No patients exhibited elevated sCD14 (>5 µg/dL)7, so as sCD14 levels did not differ by IR or correlate with HOMA-IR, no further analyses were performed on this biomarker.
Considering the previous results, we performed a stepwise logistic regression for IR with LBP, CRP, BMI and IPAQ as covariates. The regression was significant (Cox-Snell R2 = 0.397, p = 0.012), including LBP (OR = 1.203, p = 0.004), BMI (OR = 1.278, p < 0.001) and vigorous/moderate physical activity (OR = 0.242, p = 0.016). Full data available in the Supplementary material.
This is the first study to directly assess the relationship between gut permeability biomarkers and IR in schizophrenia, despite recent hypotheses about this association13. IR prevalence was high (56%), in line with the literature14, even after excluding patients with diabetes and despite the cohort’s young age. The proportion of patients with elevated LBP (37.4%) was lower than in other studies10. Our results showed elevated LBP levels despite normal sCD14 concentrations, a pattern reported in other studies9, possibly indicating an early stage of gut barrier alteration, with endotoxin exposure without sustained immune activation. Alternatively, individual variability in microbiota composition or metabolic status could influence sCD14 independently of LBP7. These observations highlight the value of assessing both markers together to better understand gut permeability.
Our findings not only align with prior evidence of gut permeability disturbances7,10 and glucose metabolism impairments14 in schizophrenia but also suggest that gut permeability may play a key role in the metabolic dysregulation observed in these patients. The association between LBP and IR persisted even after adjusting for BMI in the logistic regression analysis, highlighting gut permeability as an independent factor in glucose metabolism. Nonetheless, cross-sectional design precludes causal inference, so longitudinal or interventional studies are needed to examine their temporal relationship.
Furthermore, reduced physical activity emerged as an additional risk factor for IR in our population, consistent with literature15.
Although low-grade inflammation was initially associated with LBP (as in previous findings7), the multivariate analysis did not support definitive conclusions. This may reflect the close association between BMI and inflammatory processes16, with BMI overshadowing CRP’s independent contribution.
Overall, these findings represent a step forward in understanding the pathophysiological mechanisms underlying metabolic impairments in schizophrenia, highlighting the role of gut permeability in insulin resistance and reinforcing healthy lifestyle promotion13 as well as providing a basis for future research.
Methods
This observational, cross-sectional study is a secondary analysis of a larger project examining the role of inflammatory and gut permeability biomarkers in 146 patients with schizophrenia and bipolar disorder.
For this study, we included 91 adult, clinically stable patients diagnosed with schizophrenia according to DSM-5 criteria, without diabetes or antidiabetic treatment. Exclusion criteria addressed comorbid acute and chronic medical conditions and recent pharmacological or dietary interventions. A detailed description of these procedures, applied in the same cohort framework, has been previously published6.
Mental health clinics in Oviedo, Spain recruited the participants. The local Ethics Research Committee approved the protocol (Ref. CEImPA 2021.345).
Plasma measurements: glucose (mg/dL), insulin (μU/mL), LBP (μg/mL), C-reactive protein (CRP; mg/dL). Psychometric evaluations: Clinical Global Impression-Schizophrenia (CGI-SCH), IPAQ, Mediterranean Diet Adherence Screener (MEDAS)17. We also documented antipsychotic treatment, CPZ-ED18, toxic habits, and anthropometric measurements.
Statistical analyses used IBM SPSS v29.0. Normality was assessed with the Kolmogorov-Smirnov test. We applied Spearman’s correlation (rho), Student’s t test, Mann–Whitney U, Chi-square, and stepwise logistic regression.
Data availability
The data are available on request.
References
Nordentoft, M. et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE 8, e55176 (2013).
Greenhalgh, A. M. et al. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naïve patients with nonaffective psychosis. Schizophr. Res. 179, 57–63 (2017).
Molina, J. D., Avila, S., Rubio, G. & López-Muñoz, F. Metabolomic connections between schizophrenia, antipsychotic drugs and metabolic syndrome: a variety of players. Curr. Pharm. Des. 27, 4049–4061 (2021).
Kowalski, K. et al. Altered levels of fecal short-chain fatty acids are associated with subclinical inflammation and worse cognitive performance in patients with schizophrenia. J. Psychiatr. Res. 165, 298–304 (2023).
Safadi, J. M., Quinton, A. M. G., Lennox, B. R., Burnet, P. W. J. & Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? A systematic review and meta-analysis. Mol. Psychiatry 27, 141–153 (2022).
Paniagua, G. et al. Comparative analysis of gut microbiome-derived short-chain fatty acids in patients with severe mental disorder: Insights from schizophrenia and bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 138, 111345 (2025).
Severance, E. G. et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 148, 130–137 (2013).
Ghosh, S. S., Wang, J., Yannie, P. J. & Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 4, bvz039 (2020).
Gokulakrishnan, K. et al. Altered intestinal permeability biomarkers in schizophrenia: a possible link with subclinical inflammation. Ann. Neurosci. 29, 151–158 (2022).
González-Blanco, L. et al. Intestinal permeability biomarkers in patients with schizophrenia: additional support for the impact of lifestyle habits. Eur. Psychiatry 67, e84 (2024).
Kant, R. et al. Gut microbiota interactions with anti-diabetic medications and pathogenesis of type 2 diabetes mellitus. World J. Methodol. 12, 246–257 (2022).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395 (2003).
Zeng, C. et al. Gut microbiota: an intermediary between metabolic syndrome and cognitive deficits in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 106, 110097 (2021).
Zhuo, C. et al. Insulin resistance/diabetes and schizophrenia: potential shared genetic factors and implications for better management of patients with schizophrenia. CNS Drugs 38, 33–44 (2024).
Fernández-Abascal, B., Suárez-Pinilla, P., Cobo-Corrales, C., Crespo-Facorro, B. & Suárez-Pinilla, M. In- and outpatient lifestyle interventions on diet and exercise and their effect on physical and psychological health: a systematic review and meta-analysis of randomised controlled trials in patients with schizophrenia spectrum disorders and first episode of psychosis. Neurosci. Biobehav. Rev. 125, 535–568 (2021).
Van Dyne, A. et al. Longitudinal relationships between BMI and hs-CRP among people with schizophrenia. Schizophr. Res. 271, 337–344 (2024).
Martínez-González, M. A. et al. A 14-item mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS ONE 7, e43134 (2012).
Gardner, D. M., Murphy, A. L., O’Donnell, H., Centorrino, F. & Baldessarini, R. J. International consensus study of antipsychotic dosing. Am. J. Psychiatry 167, 686–693.
Zweigner, J., Gramm, H.-J., Singer, O. C., Wegscheider, K. & Schumann, R. R. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 98, 3800–3808 (2001).
Acknowledgements
This study was supported by a grant from the Ministry of Economy and Competitiveness the Instituto de Salud Carlos III (ISCIII, grant number PI21/01393) and co-funded by the European Union, CIBER – Consorcio Centro de Investigación Biomédica en Red - (CB/07/09/0020); and by the Government of the Principality of Asturias ref. IDE/2024/774; and by a grant from the Foundation for Research and Biosanitary Innovation in the Principality of Asturias (FINBA) “Convocatoria Intramural para el Fomento de Proyectos de Investigación entre Investigadores Nóveles - Ref. GOBLL/2021-051-INTRAMURAL NOV-GOBLL”, sponsored by Janssen.
Author information
Authors and Affiliations
Contributions
L.B.G. and M.P.G.P. designed the project. L.G.B., P.A.S., and M.P.G.P. coordinated the project development. M.C.S. drafted the manuscript. F.D.S., L.G.B., G.P., J.R.R., P.A.S., and M.P.G.P. participated in the recruitment. M.C.S., L.G.B., and M.P.G.P. performed the statistical analyses. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.G.B. has been a consultant to and/or has received lecture fees/grants from the Spanish Foundation of Psychiatry and Mental Health, Otsuka, Lundbeck, Johnson & Johnson, Casen Recordati, Angelini and Pfizer. G.P. has been a consultant to and/or has received honoraria or grants from Adamed, Alter Medica, Angelini Pharma, Johnson & Johnson, Lundbeck, Otsuka and Viatris España. P.A.S. has been a consultant to and/or has received honoraria or grants from Adamed, Alter Medica, Angelini Pharma, CIBERSAM, Ethypharm Digital Therapy, European Commission, Government of the Principality of Asturias, Instituto de Salud Carlos III, Johnson & Johnson, Lundbeck, Otsuka, Pfizer, Plan Nacional Sobre Drogas, Rovi, Servier and Viatris España. M.P.G.P. has been a consultant to and/or has received honoraria/grants from Angelini, Otsuka-Lundbeck Alliance, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Otsuka, and Pfizer. The other has no competing interests. All other authors declare that they have no conflicts of interest related to the current work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Couce-Sánchez, M., Dal Santo, F., González-Blanco, L. et al. Insulin resistance in patients with schizophrenia: is it related to gut permeability?. Schizophr 11, 145 (2025). https://doi.org/10.1038/s41537-025-00688-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41537-025-00688-w