Abstract
Oolong tea flavor is shaped by metabolite variations induced by season, processing, and cultivar. This study investigated the metabolic basis of sensory characteristics in Yongchun Foshou (YCFS) tea by integrating HS-SPME/GC-MS, HPLC, quantitative descriptive analysis (QDA), and chemometrics. Orthogonal partial least squares discriminant analysis (OPLS-DA) pinpointed key differential compounds between spring (YCFS-S) and autumn (YCFS-A) teas. Two-way ANOVA with effect size analysis confirmed that season was the dominant factor influencing most metabolites, although grade was the primary driver for free amino acids. YCFS-S contained significantly higher levels of taste-related phytochemical compounds, whereas YCFS-A exhibited elevated tea polyphenols. Statistical sensory analysis further identified a subset of key aroma compounds as drivers of seasonal and grade-based aroma differences. Correlation network analysis pinpointed 27 key sensory drivers: 12 aroma compounds defining a complex aroma profile dominated by floral and herbal notes, and 15 phytochemical compounds responsible for the umami, overall richness, and balanced bitterness. These findings were synthesized to construct the first dedicated flavor wheel for YCFS, elucidating how seasonality and grade collectively shape its flavor profile and providing a scientific basis for quality control and standardized production.
Similar content being viewed by others
Introduction
Tea (Camellia sinensis) is one of the world’s three major nonalcoholic beverages and is an important component in various cultures1. The market value and consumer acceptance of tea are largely determined by its quality, which depends on factors such as cultivar, growing environment, processing techniques, and harvest season2,3,4. Among these factors, seasonal variation plays a pivotal role in shaping the metabolic profile and sensory characteristics of finished tea, thereby ultimately determining its quality grade5. Fresh tea leaves contain diverse bioactive compounds, such as polyphenols, amino acids, volatiles, and alkaloids, which form the quality foundation of tea by contributing to both its potential biomedical applications and desirable flavor profiles6. Seasonal changes in environmental conditions considerably impact the accumulation of these metabolites. Specifically, spring teas are characterized by enhanced nitrogen metabolism, which promotes the accumulation of amino acids and certain flavor compounds, whereas autumn teas undergo strengthened carbon metabolism, leading to higher levels of aroma compounds and polyphenols7. Oolong tea, a semi-fermented type of tea, is typically produced in spring and autumn8. These differential metabolic pathways contribute distinctly to the sensory attributes of the final product: spring oolong teas are often characterized by a delightful and mellow taste, while autumn teas are renowned for their rich and pleasant aroma8. These differences have been well documented in famous oolong teas such as ‘Tieguanyin’, which shows clear seasonal variations in metabolite composition consistent with traditional harvesting practices9.
The discernible differences in tea quality are formally categorized in the market through a grading system. Tea quality grading, guided by the sensory evaluation methodology of Chinese tea (GB/T 23776-2018, GB/T: National Standards of the People’s Republic of China), reflects the underlying metabolite composition and sensory traits. High-grade oolong teas exhibit greater mellowness, lightness, and lower bitterness, shaped by environmental and processing factors10. Grading considers leaf appearance, liquor color, aroma, taste, and brewed leaf condition, with superior grades commanding higher market value. Oolong tea is typically classified into superior grade, first grade, second grade, and third grade. Despite constituting only a small fraction of the dry weight, aroma volatiles are particularly crucial as they significantly influence consumer preference and economic value11.
Yongchun Foshou oolong tea (YCFS) is a geographically indicated product from Yongchun County, Fujian Province. This distinctive tea is produced from the large-leaf ‘Foshou’ cultivar of C. sinensis var. sinensis. It is characterized by its unique Buddha’s hand-shaped leaves and is renowned for its rich aroma and mellow flavor. YCFS represents a significant and valuable segment of the Fujian oolong tea industry. While earlier studies have touched upon roasting techniques, storage conditions, and potential health benefits of YCFS12, several key aspects remain underexplored. A comprehensive profile of its aromatic characteristics is still lacking, and the effects of season on flavor compounds and their relationship with quality grading are not well understood. The potential interaction between season and grade also awaits exploration.
To address these gaps, we combined quantitative descriptive analysis (QDA) with high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC–MS) to quantify nonvolatile and volatile metabolites. The compounds were examined for their considerable contribution to YCFS and the flavor properties of YCFS using orthogonal partial least squares variable importance projection (OPLS-VIP), partial least squares VIP (PLS-VIP), and odor activity value (OAV) parameters. The objectives of this study were as follows: (1) to construct the first dedicated flavor wheel for Yongchun Foshou oolong tea by characterizing its key aroma and taste compounds, (2) evaluate the impact of seasonal variations on its quality by analyzing metabolic changes and identifying key seasonal discriminators, and (3) identify the key flavor compounds that influence YCFS grades, with specific attention to the interplay between harvest season and grade differentiation. These findings will help determine the seasonal quality characteristics and flavor compounds that affect quality grade differences, providing insights for high-quality oolong tea production.
Results
Sensory evaluation of YCFS
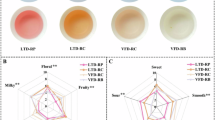
To investigate the impact of season and grade on the sensory quality of YCFS, 40 samples were selected. The samples were classified as YCFS-S (spring) and YCFS-A (autumn), labeled by grade (SA-SD for spring, AA-AD for autumn; Table 1). Overall, YCFS is characterized by its excellent organoleptic qualities, featuring an orange–yellow and clear infusion color, a mellow taste, and strong, persistent floral and fruity aromas. These attributes collectively define the overall flavor profile of YCFS, as evaluated via sensory analysis in accordance with GB/T 23776-2018.
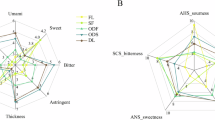
Significant differences were observed between the samples of different seasons and grades (Fig. 1, Supplementary Tables 1–3). YCFS-S and YCFS-A exhibited distinct taste and aroma profiles. In terms of sensory profile, YCFS-S delivered a richer umami taste and subtle aroma, whereas YCFS-A developed pronounced floral-woody notes with a rich mouthfeel that is slightly astringent and bitter. YCFS-S scored higher in sweetness, mellowness, and umami, whereas YCFS-A had higher scores for bitterness and astringency. YCFS-S displayed subtle fruity and herbal notes, whereas YCFS-A was dominated by floral, sweet, and woody aromas. The A-grade samples in both YCFS-S and YCFS-A stood out demonstrating the highest scores in mellowness (6.30, 5.78) and umami (6.36, 5.74), followed by grade B (mellowness: 6.10, 5.34; umami: 5.76, 4.58), grade C (mellowness: 5.56, 4.96; umami: 5.54, 4.36), and grade D (mellowness: 5.02, 4.56; umami: 4.80, 4.12). In addition, the sweetness and astringency attributes of the YCFS-S samples were ranked from highest to lowest according to grade, whereas YCFS-A showed a clear descending order in floral and herbal aromas by grade.
a Taste evaluation of four grades of YCFS-S. b Aroma evaluation of four grades of YCFS-S. c Taste evaluation of four grades of YCFS-A. d Aroma evaluation of four grades of YCFS-A. The dark purple circle denotes grade A; the pink circle, grade B; the orange circle, grade C; and the blue circle, grade D. e Sensory evaluation of YCFSs.
Characteristic phytochemical compounds
The flavor profile of tea, characterized by sweetness, mellowness, freshness, bitterness, and astringency, is formed by water-soluble compounds, including amino acids, polyphenols, caffeine, soluble sugars, and tea pigments. Caffeine, a prominent alkaloid, confer a characteristic bitterness that intensifies the perceived strength of the tea. Of the many flavor compounds, amino acids, which are key contributors to the umami and sweet taste of tea, are typically more abundant in spring tea. The amino acid levels in our samples ranged from 0.62% to 2.61%, which falls within the typical amino acid range for tea13. Soluble sugars, ranging from 6.63% to 17.48%, play a pivotal role in balancing bitterness and enhancing sweetness, markedly impacting tea quality. Catechins, including C (0.27–1.20 mg/g), GC (0.68–3.97 mg/g), EC (1.48–11.01 mg/g), EGC (4.40–22.94 mg/g), ECG (1.38–26.07 mg/g), and EGCG (10.04–38.73 mg/g), are key contributors to the astringency and bitterness of oolong tea. Esterified catechins such as EGCG and ECG are particularly associated with strong astringency, whereas nonesterified forms such as EGC and EC provide milder astringency and subtle sweetness14. TFs (0.06–0.12%), TRs (0.10–0.59%), and TBs (0.60–3.35%) also contribute to flavor, with TFs adding a slight briskness15. Compared to some previous reports16,17, the polyphenol content in our samples (7.12–9.49%) appears relatively low, which may be attributed to various factors such as tea plant varieties, processing methods, and harvesting standards. Total flavonoids are important antioxidant constituents in tea and usually constitute 5.00%–10.00% of the dry weight of tea leaves18. The flavonoid content in YCFS ranged from 7.57% to 15.03% (Fig. 2), consistent with previous reports indicating higher flavonoid levels in ‘Foshou’ cultivar19.
a Content of the main nonvolatile chemical constituents; (b) content of the six catechin monomers. c OPLS-DA score plot of phytochemical compounds in YCFSs from different seasons. d PLS-DA score plot of phytochemical compounds in YCFSs from different grades. e Variable importance in projection (VIP) values distinguishing the phytochemical compounds of YCFS-S and YCFS-A. f VIP values distinguishing phytochemical compounds across different YCFS grades. Data are presented as mean ± SD (n = 5). Red indicates SA samples; tangerine, SB samples; light orange, SC samples; yellow, SD samples; cyan, AA samples; sky blue, AB samples; dark blue, AC samples; and light purple, AD samples. For phytochemical compounds without significant interaction effects, mean separation was performed according to Tukey’s HSD test. For compounds with significant interaction effects, simple effect analysis was conducted. Mean separation within each fixed season was performed using Bonferroni’s post-hoc test. Different uppercase letters (A, B, and C) indicate significant (p < 0.05) sample differences among the four grades of YCFS-S; different lowercase letters (a, b, and c) indicate significant (p < 0.05) sample differences among the four grades of YCFS-A.
Impact of season and grade on phytochemical compounds
To determine the main factors influencing the phytochemical composition of YCFS, a two-way ANOVA was performed using harvest season and quality grade as independent variables (Supplementary Table 4). The results showed that season had a dominant effect on most phytochemical compounds. For example, tea polyphenols (partial η² = 0.936), caffeine (partial η² = 0.846), and EC (partial η² = 0.978) were predominantly influenced by season, which accounted for over 84% of the variance in these components. In contrast, the amino acid content was primarily driven by grade (partial η² = 0.834), making it a key chemical marker for quality discrimination. Significant interactive effects between season and grade were also observed for several compounds, including amino acids, tea polyphenols, TFs, TBs, C, EGC, and EGCG, indicating that their levels depend on the combination of both factors. Simple effects analyses were conducted to further explore these interactions (Supplementary Tables 5 and 6).
Comparative analysis revealed notable seasonal differences in phytochemical composition (Fig. 2, Supplementary Tables 7 and 8). Specifically, YCFS-S showed higher levels of amino acids, flavonoids, caffeine, C, and GC, whereas YCFS-A had higher levels of tea polyphenol, TF, TR, TB, and select catechins (EC, EGC, ECG, and EGCG). Grade-related variations were also evident. In YCFS-S, significant differences across grades were observed for amino acids, tea polyphenols, and TRs, whereas these differences were less pronounced in YCFS-A (Fig. 2). The chemical properties of tea samples exhibit significant variations across different grades. Specifically, lower-grade teas show elevated concentrations of TFs, TRs, C and EGCG. Conversely, higher-quality teas demonstrate increased levels of amino acids, tea polyphenols, and EGC. Multivariate analyses (OPLS-DA and PLS-DA) further confirmed significant compositional differences between seasons and more subtle variations among grades (Fig. 2c, d, Supplementary Fig. 1). Based on variable importance in projection (VIP > 1), 12 key phytochemicals were identified as differentiating between YCFS-S and YCFS-A, including tea polyphenols, flavonoids, caffeine, TFs, TRs, and individual catechins. For grade discrimination, the main distinguishing compounds were free amino acids, soluble sugar, tea polyphenols, and TFs (Fig. 2e, f).
Taste-chemical correlation profiling
Correlation analysis revealed marked associations between the phytochemical components and sensory attributes of YCFS (Fig. 3a), thereby elucidating the chemical basis of its characteristic flavors. Sweetness and umami were strongly associated with amino acids. Astringency and bitterness were predominantly influenced by epigallocatechin (EGC), epicatechin (EC), esterified catechins (EGCG, ECG), and flavonoids. These correlations strongly indicate that the characteristic umami and sweetness of YCFS-S should originate from its significantly higher abundance of free amino acids, a finding consistent with its superior sensory scores for these attributes. Conversely, the robust and more astringent profile of YCFS-A is likely derived from its enriched pool of the aforementioned phenolic compounds. The perception of astringency primarily arises from the interaction of these compounds (C, GC, EC, EGC, EGCG, ECG) with salivary proteins, creating a dry, puckering sensation20. Bitterness was positively correlated with caffeine, tea polyphenols, GC, C, TRs, and TBs. Notably, the higher tea polyphenol content in YCFS-A likely constitutes the primary source of bitterness. Furthermore, thickness exhibits a strong positive correlation with water extract content, as well as with GC, C, TBs, TRs, and tea polyphenols. This indicates that the thickness attribute of YCFS is not driven by a single compound but arises from a harmonious and complex richness in the mouth, resulting from the combined contribution of various water-soluble components. Applying thresholds of VIP > 1, p < 0.05, and |r | >0.5 identified water extract, caffeine, flavonoids, tea polyphenols, C, GC, EC, EGC, EGCG, ECG, TRs, and TBs as critical variables defining YCFS’s taste signature, which was in alignment with recognized flavor compounds of oolong tea.
a Correlation between the phytochemical compounds and taste attributes of YCFS. b Correlation between the aroma compounds and aroma attributes of YCFS. c Correlation network between taste sensory attributes and phytochemical compounds. d Correlation network between olfactory sensory attributes and aroma compounds. Nodes represent sensory attributes (rhombus) and chemical components (circles). Edges (lines) represent significant correlations ( | r | > 0.6, p < 0.05). The color of the edges correspond to the strength of the Pearson correlation coefficient, with solid red lines indicating positive correlations and blue lines indicating negative correlations.
Characterization of aroma compounds
Aroma plays a pivotal role in the evaluation of tea quality. The complexity of aromatic compounds has attracted considerable interest in tea aroma assessment. We employed HS-SPME coupled with GC-MS to analyze aroma compounds. A total of 214 aroma compounds were identified, which were classified into 10 categories based on the nature of functional groups, including 39 esters, 33 terpenes, 31 alcohols, 30 aldehydes, 27 ketones, 27 hydrocarbons, 10 acids, 9 aromatics, 7 heterocyclic compounds, and 2 ethers (Supplementary Table 9). The average relative content of terpenoids in YCFSs was 5.33 μg/g, followed by alcohols at 1.51 μg/g, esters at 1.25 μg/g, ketones at 1.17 μg/g, aldehydes at 0.93 μg/g, hydrocarbons at 0.38 μg/g, heterocyclic compounds at 0.24 μg/g, aromatics at 0.07 μg/g, and acids at 0.004 μg/g. Tea aroma is determined by its endogenous aroma compounds, with their ratio defining their unique characteristics. Statistical analysis revealed that only the proportions of terpenoids and ketones in YCFSs exhibited significant differences between the two seasons (Fig. 4b, Supplementary Table 9). Terpenoids, with lower odor detection thresholds and pleasant sensory attributes, play a pivotal role in forming the aroma profile of tea21. Ketone accumulation is also a characteristic of high-quality aromatic oolong teas22. This suggests that terpenoids and ketones may play a pivotal role in the formation of the YCFS flavor profile. Several oolong tea characteristic aroma compounds were identified among the 214 volatiles detected in YCFS. These include terpene-derived compounds such as (E)-nerolidol, citral, and α-terpineol; amino acid-derived volatiles such as benzaldehyde, benzyl alcohol, and indole; fatty acid–derived volatiles including hexanal, heptanal, and jasmine lactone. In addition, (E)-nerolidol, nerol oxide, linalool, geraniol, α-ionone, and α-farnesene, which contribute to the quality suitable for oolong tea processing, were also prevalent in the YCFS samples. These aromatics have distinctive floral and fruity notes that make up the aromatic flavor profile of YCFS.
a Category and quantity of volatile compounds for all samples. b The ratio of aroma compounds. c OPLS-DA score plot of aroma compounds in YCFSs from different seasons. d PLS-DA score plot of aroma compounds in YCFSs from different grades. e Venn diagram of the main aroma compounds (VIP > 1) distinguishing YCFS by season and grade. f Heat map of aroma compounds (VIP > 1, p < 0.05) distinguishing YCFS by season and grade.
Impact of seasonal and graded variations on YCFS fragrance profile
Seasonal variations regulate the accumulation of aroma compounds in tea leaves, primarily through enhanced nitrogen metabolism during spring that supplies precursors for key aroma volatiles23. We focused only on aroma compounds that were detected in more than 50% of the samples across all groups for the subsequent multivariate analysis to reduce data dimensionality and focus on the most consistently present volatiles. Multivariate analyses (OPLS-DA and PLS-DA) effectively discriminated aroma profiles between seasons and quality grades (Fig. 4c, d), with model validation confirming robustness and no overfitting (Supplementary Fig. 1). Variable importance in projection (VIP > 1) analysis identified 30 and 31 aroma compounds as markers for seasonal and grade differentiation, respectively, with 20 compounds common to both comparisons, suggesting their dual role in distinguishing both seasonal and grade variations (Fig. 4e, Supplementary Table 10, Supplementary Fig. 2). Effect size analysis, based on partial η² values from the two-way ANOVA, provided further insight into the relative influence of each factor. For the fourteen key aroma compounds identified the effect of season (partial η² > 0.14) was consistently greater than that of grade, indicating that seasonal variation was the dominant source of variance in the abundance of these aroma compounds (Supplementary Table 4). However, a critical “season × grade” interaction was observed. Results revealed that the discrimination of tea grades was predominantly established in YCFS-S, whereas YCFS-A samples showed markedly less grade-based separation (Supplementary Table 6). This indicates that while season sets the foundational aroma profile, the opportunity to refine and distinguish quality through grade is significantly more pronounced in spring teas than in autumn teas. Key aroma compounds such as linalool (Floral), 3,5-octadien-2-one (Fruity), α-ionone (Floral, Woody), 2-heptanone (Fruity, Cheesy), and 1-octen-3-one (Mushroom) exhibited extremely strong seasonal effects (partial η² > 0.79), indicating that their formation is highly dependent on climatic conditions. Notably, while these compounds have been individually reported in other oolong teas, their collective dominance and exceptionally high seasonal specificity in YCFS represent a unique aromatic profile. Specifically, the synergistic presence of linalool and α-ionone at such high concentrations in YCFS-S emphasizes floral and sweet notes. Combined with the distinct fruity tones imparted by 2-heptanone and 3,5-octadien-2-one, this distinguishes YCFS from known oolong varieties, indicating that its unique aroma profile is often regulated by seasonal cues. In contrast, the grade showed a significant main effect on fewer aroma compounds. The most notable exception was 2-nitroethylbenzene (Floral), for which grade was the primary source of variation (partial η² = 0.492). The prominent performance of 2-nitroethylbenzene demonstrates that it provides a unique set of graded markers for YCFS. While 2-nitroethylbenzene has been reported as a volatile in teas, it is rarely emphasized as a key grade marker in premium oolongs like ‘Tieguanyin’ or ‘Dahongpao’, where compounds such as nerolidol, indoles, or specific terpenoids typically define quality24,25,26. This widespread interaction indicates that the expression of a tea’s grade-specific aroma signature is strongly season-dependent. To unravel these complex relationships, simple effects analyses were conducted. The results revealed that the significant differences in key aroma compounds between grades were predominantly established in YCFS-S, while YCFS-A samples showed less grade-based discrimination (Fig. 4d, Supplementary Table 6). Hierarchical clustering analysis was conducted to further evaluate aroma compound variations, and the results were visualized using heat maps (Supplementary Table 7). Among the main compounds identified to distinguish the seasons, (E, E)-2,4-heptadienal, linalool, and other chain-structured compounds with floral aromas were found in higher abundance in YCFS-S than in YCFS-A27. Notably, YCFS-A exhibits elevated concentrations of volatile compounds, including (E)-nerolidol, geraniol, and α-ionone. Within the context of oolong tea, (E)-nerolidol is frequently associated with woody and herbal aromas, geraniol imparts a floral (rose-like) fragrance, and α-ionone contributes violet-like and sweet notes28,29,30. The elevated levels of these compounds in YCFS-A may collectively explain its significantly higher sensory scores for “herbal,” “woody,” and “sweet” aroma attributes, suggesting their potential role in defining the unique aromatic profile of this YCFS-A.
Grade-related patterns revealed that premium grades (SA, SB, AA) contained higher levels of (E)-β-farnesene and jasmone, associated with woody and floral notes31. Lower-grade samples contained more (E)-2-decenal and 2-hexenal, imparting green and fatty aromas, potentially indicative of lighter processing approaches. These chemical patterns correspond with sensory evaluation results and provide mechanistic insights into quality variations in YCFS.
To further explore the specific contributions of these aroma compounds to the flavor profile of YCFS, we calculated the OAVs (Table 2), and visualized the concentration differences using a heat map (Fig. 4f). Linalool and phenylacetaldehyde exhibited the highest concentrations in YCFS-S and YCFS-A, with the former providing a floral scent and the latter conferring honey and sweet floral aromas. These compounds are key to various teas, particularly oolong tea31,32. The higher abundances of linalool, nonanal, and phenylacetaldehyde in YCFS-S result from seasonal biosynthetic changes. Autumn’s intense light stress induces a photoprotective response, increasing the accumulation of compounds like linalool and carotenoids33. The subsequent conversion of carotenoids into aroma compounds like α-ionone demonstrates the seasonal dependence of oolong tea’s aroma formation and the development of its distinctive flavor.
Key aroma compounds and aroma-sensory correlation profiling in the YCFS
Odor threshold constitutes a critical consideration in elucidating compound-sensory perception relationships34. Typically, OAV is used to validate the relative contribution of active odor compounds and aroma compounds, effectively identifying key aroma compounds35. Through integrated OPLS-DA/PLS-DA modeling and OAV analysis (VIP > 1, OAV > 1), we identified 12 aroma compounds: linalool, 2-nitroethylbenzene, 3,5-octadien-2-one, α-ionone, hexanal, heptaldehyde, 1-octanol, 1-octen-3-ol, nonanal, benzaldehyde, dihydroactinidiolide, and phenylacetaldehyde (Table 2). Notably, linalool and α-ionone respectively characterize the floral notes of YCFS-S and YCFS-A.
To elucidate the flavor profiles of key aroma compounds in YCFS, Pearson correlation analysis was conducted (Fig. 3b). The floral attribute showed significant positive correlations with linalool, α-ionone, 1-octen-3-ol, 1-octanol, and dihydroactinidiolide. The strong positive correlation between the floral attribute and linalool/α-ionone is highly consistent with their well-established olfactory profiles. The association of linalool with fresh, sweet floral notes and α-ionone with violet-like and woody aromas is consistent with the sensory characteristics of YCFS-S and YCFS-A, respectively36,37. The fruity attribute represents a notable exception, demonstrating a significant positive correlation only with linalool. Sweet was positively correlated with linalool, 2-nitroethylbenzene, dihydroactinidiolide, 1-octen-3-ol, α-ionone, and phenylacetaldehyde. Woody/herbal attributes correlated with dihydroactinidiolide, α-ionone, 1-octen-3-ol, and 1-octanol. Notably, negative correlations were observed between nearly all sensory attributes and the concentrations of hexanal, heptaldehyde, and nonanal. Analysis of their distribution across quality grades revealed that these aldehydes were significantly more abundant in lower-grade samples. As established markers of fatty acid oxidation38, their elevated levels primarily indicate the use of less fresh or more mature raw materials, which are inherently more susceptible to oxidative damage during harvesting and initial processing. Additionally, potential sensory antagonism or masking effects may also contribute to these findings. Several compounds, such as linalool and α-ionone, correlated with multiple attributes, indicating their role as core components of the YCFS flavor profile. Overall, the correlation analysis supports the OAV findings and provides a sensory-based interpretation of the perceptual differences between YCFS-S and YCFS-A.
The flavor wheel construction of YCFS
A flavor wheel is a tool used to categorize and organize sensory attributes, helping to intuitively describe the flavor profile of food39. By combining QDA with the flavor wheel, both the flavor characteristics and their visualization can be achieved. The Chinese Academy of Agricultural Sciences Tea Research Institute has developed the original terminology and flavor wheel for Chinese tea sensory evaluation40. In this study, the flavor characteristics of YCFS were systematically described and compared to optimize product quality control and explore factors affecting flavor variation (Fig. 5). The YCFS flavor wheel incorporates 12 sensory attributes and 27 chemical compounds identified through QDA, and employs chemometric methods to select the 12 key chemical compounds and 10 aroma compounds that make important contributions to each sensory attribute (Fig. 3c, d). The compounds in the third level of the flavor wheel contributed significantly to sensory sub-attributes at the second level (p < 0.05), clearly illustrating the core flavor characteristics of YCFS and their chemical underpinnings.
In terms of aroma attributes, six components showed significant positive correlations with herbal notes, five with sweet aroma, two with floral, and one each with fruity, woody, and green characteristics. Each of these compounds displayed the highest correlation coefficient within its respective aroma attributes. The sensory chemistry of the YCFS flavor wheel reveals distinct chemical drivers for its core attributes compared to existing oolong wheels. For instance, the prominent “fruity” note in many oolongs like ‘Shuixian’ is primarily attributed to compounds such as geraniol, indole, and phenylethyl alcohol41. In contrast, our research indicates that the less pronounced fruity character in YCFS is largely driven by linalool, a compound that typically imparts floral notes in other teas but here appears to contribute to both floral and fruity aromas simultaneously. Furthermore, the prominent floral, woody, and herbal notes in YCFS, driven by (E)-nerolidol, α-ionone, 1-octen-3-ol, and 2-nitroethylbenzene, constitute its core aromatic attributes. These stand in stark contrast to the predominant “fruity” and “roasted” flavors featured in the flavor wheels of many other oolong teas42. This reprioritization of attributes, which elevates “herbal” and “woody” from peripheral to central positions, effectively redefines the sensory hierarchy for YCFS.
Regarding taste attributes, it is well established that caffeine and tea polyphenols produce bitterness, while GC and C contribute to both bitterness and astringency43. In the YCFS profile, these components are indeed recognized as the primary sources of bitterness. Similarly, the substances primarily responsible for astringency, including flavonoids, EC, EGC, ECG, and EGCG—are identified as the astringent compounds in YCFS. Water extracts serve as key substances for thickness perception. Umami and sweetness are both attributed to amino acids, aligning with their inherent flavor profiles and thereby validating the fundamental taste wheel of YCFS. Interestingly, although flavonoids and TFs are typically associated with astringency, our analysis found them to be positively correlated with mellowness in YCFS as well. While this relationship is weak, it suggests that the flavor profile of YCFS differs from traditional oolong teas, where such a connection is rarely emphasized. The hydrolysis of flavonoids into aglycones and sugars may mitigate their astringency, effectively transforming them into positive contributors to mellowness. This suggests a unique mechanism for regulating astringency in YCFS. These associations collectively delineate a flavor profile for YCFS that diverges from the typical oolong spectrum: it possesses a more pronounced herbal-woody backbone complemented by a balanced sweet-floral overlay. This divergence not only reflects the cultivar’s unique metabolic wiring but also offers a new framework for understanding the diversity of oolong tea flavor landscapes.
Discussion
Tea plant metabolism is highly susceptible to seasonal variations, which lead to marked differences in the quality of finished teas across harvest seasons. Generally speaking, spring tea are considered to be of higher quality than autumn tea7. Our findings support this notion, demonstrating that YCFS-S contains elevated levels of numerous compounds associated with desirable flavor characteristics. In this study, we utilized an OPLS-DA model to effectively discriminate between YCFS-S and YCFS-A based on their distinct phytochemical and aroma profiles. Furthermore, two-way ANOVA with effect size estimation demonstrated that seasonal variation serves as the primary source of metabolite differences, accounting for the majority of variance (partial η² > 0.14) in the measured compounds. The superior umami and sweetness of YCFS-S are mechanistically explained by its significantly elevated amino acid content. In contrast, the stronger astringency and bitterness in YCFS-A are attributed to higher concentrations of tea polyphenols and catechins14. It aligns with previous research findings: spring teas accumulate more amino acids, while autumn teas accumulate more polyphenols44. Flavonoid variation is also influenced by season. Its synthesis is likely inhibited by reduced UV-B exposure in autumn45. This reduction may contribute to a smoother mouthfeel, as bitterness is reduced through hydrolysis46.
Beyond determining flavor, seasonality exerts a profound impact on the aroma composition of tea, altering its underlying chemical foundation. YCFS-S contains higher levels of linalool and phenylacetaldehyde. These compounds contribute floral-fruity and sweet aromas in black tea47. They are likely the source of the floral and fruity notes in YCFS-S. In contrast, YCFS-A accumulates higher levels of 3-octen-2-one, α-ionone, benzaldehyde, and dihydroactinidiolide. These compounds play a critical role in the formation of oolong tea’s aroma48. They likely give YCFS-A its distinctive floral and woody character. This divergence in aroma profiles is likely driven by the distinct climatic conditions of each season. We speculated that, similar to previous studies, intense spring sunlight may reduce the ability to convert linalool into linalool oxides, leading to linalool accumulation49. Phenylacetaldehyde, a degradation product of the amino acid phenylalanine, aligns with the higher amino acid accumulation in spring teas50. Notably, the accumulation of α-ionone and dihydroactinidiolide points to increased carotenoid degradation in YCFS-A51. Benzaldehyde, potentially derived from further oxidation of benzyl alcohol or phenylalanine, also contributes to the warmer, woody-almond notes characteristic of autumn teas31. The seasons fundamentally shape the flavor profile of YCFS by influencing the metabolism of tea plants, thereby determining its fundamental flavor framework.
Beyond seasonal influences, tea grade emerges as a sophisticated refining factor governing specific quality parameters. Two-way ANOVA revealed significant main effects of both season and grade on the accumulation of free amino acids. The effect size for grade (partial η² = 0.83) was larger than that for season (partial η² = 0.42), indicating that grading standards are the dominant factor governing amino acid content in YCFS. This suggests that while the seasonal growing environment sets a baseline for amino acid synthesis, human-controlled practices associated with high-grade production, such as the selective plucking of tender buds and optimal processing, are exceptionally effective at maximizing and preserving these desirable compounds. A similar pattern was observed for 2-nitroethylbenzene and heptaldehyde, further supporting the dominant role of grade in regulating certain constituents. Correlation analysis confirmed that higher-grade teas intensified the desirable attributes associated with their season: umami and sweetness became more prominent in spring teas, while the floral-woody notes were enhanced in autumn teas. Meanwhile, the levels of bitterness- and astringency-related compounds were better controlled in higher-grade teas, resulting in a more balanced and harmonious taste profile. Therefore, tea grades amplify the positive sensory attributes derived from seasonal growing conditions, thereby enhancing the overall quality of the tea. By integrating these chemometric findings with sensory evaluation, we have constructed the first flavor wheel for YCFS.
While the correlations established in this study provide a robust and valuable framework for understanding YCFS flavor, it is crucial to contextualize these findings within the methodological scope of the work. The identification of high-OAV compounds as key aroma drivers, while strongly supported by our statistical models, remains an inferential conclusion that necessitates causal validation. The primary limitation lies in the nature of the OAV itself: it is a theoretical potency index that does not account for the complex physicochemical interactions within a real food matrix, such as the partitioning of volatiles between lipid and aqueous phases. Consequently, a compound with a high OAV might see its perceived intensity diminished due to matrix binding, or conversely, its contribution could be overestimated if its characteristic aroma is masked or altered in the complex mixture. Furthermore, the proposed synergistic effects, though plausible from a chemometric standpoint, are currently hypothetical constructs derived from correlation; without functional validation, their true sensory impact and the very identity of the primary drivers cannot be definitively confirmed. To bridge the gap between relevant inferences and causal understanding, future research should explicitly target molecular sensory validation. A critical next step involves adopting reconstitution and omission experiments. This approach not only confirms the necessity of each quality-determining component but also enables empirical verification of hypothesized synergistic interactions. Building upon this foundation, specific unresolved interactions can be addressed—such as investigating how environmental factors like altitude, soil, and microclimate influence the accumulation of YCFS quality components. This study systematically demonstrated the seasonal and grade-dependent flavor signatures of YCFS by integrating chemometric modeling (OPLS-DA/PLS-DA), analysis of variance (two-way ANOVA), and correlation analysis. Several key findings emerge: first, the flavor profile of YCFS is predominantly governed by seasonality, with YCFS-S distinguished by umami taste and delicate floral aromas, underpinned by a higher abundance of amino acids and linalool. In contrast, YCFS-A exhibits intensified floral-fruity and bitter notes, a consequence of elevated levels of carotenoid-derived volatiles and polyphenols. Second, tea grade serves as a crucial refining factor. Higher-grade samples (A/B) consistently retained more key aroma compounds and demonstrated superior sensory performance, attributable to superior raw material. Finally, correlation analysis definitively linked specific flavor compounds to sensory attributes: amino acids to sweetness and umami, esterified catechins (EGCG, ECG) to bitterness and astringency, while a suite of aroma compounds correlates with multiple aroma attributes rather than a single aroma attribute. For instance, linalool simultaneously enhanced floral and fruity notes, while α-ionone simultaneously boosted floral and sweet aromas. This highlights the importance of season and grade in regulating tea quality. To build upon these findings, future research should address specific, unresolved interactions, such as “investigating the effects of environmental factors like altitude, soil, and microclimate on the accumulation of quality components in YCFS” or “examining the role of green ecological cultivation practices in enhancing YCFS quality” to elucidate environmental-varietal interactions
Methods
Chemical reagents
Standards of (−)-catechin (C), ( − )-epicatechin (EC), ( − )-gallocatechin (GC), ( − )-epicatechin gallate (ECG), ( − )-epigallocatechin (EGC), ( − )-epigallocatechin gallate (EGCG), and caffeine were purchased from Sigma-Aldrich Co. Ltd (St. Louis, MO, USA), at a purity of 99.0%. Liquid chromatographic (LC) -mass spectrometry (MS)-grade acetonitrile, methanol, acetic acid, and ethyl caprate (purity ≥ 98.0%) were purchased from Shanghai Aladdin Biological Co., Ltd (Shanghai, China). Ethanol (chromatographic grade) and ascorbic acid (analytical grade) were purchased from Shanghai Sinopremedy Chemical Reagent Co., Ltd. (Shanghai, China). Ethyl decanoate (internal standard) was purchased from Sigma-Aldrich Fluka Co., Ltd. (St. Louis, MO, USA). The normal-alkane mixture was acquired from Aladdin Biochemical Technology Co., Ltd. (C7-C40 linear alkanes, Shanghai, China).
Tea sample collection
All 40 samples of Yongchun Foshou oolong tea (YCFS) were selected and collected from the 2023 spring (YCFS-S) and autumn (YCFS-A) championships of the King of the Tea in Yongchun County, Fujian Province, China. Trained experts classified the samples into four grades: superior, first, second, and third grades, represented by A, B, C, and D, respectively. The YCFS-S samples included SA, SB, SC, and SD, whereas the YCFS-A samples included AA, AB, AC, and AD. To reduce variability, five biological replicates (500 g) were randomly collected for each grade in each season. All samples were sealed in high-barrier aluminum foil bags and stored at 4°C in a light-protected environment until metabolite analysis, which was conducted upon completion of collection. Sample details are presented in Table 1.
Sensory evaluation
The sensory evaluation of tea samples was conducted according to GB/T 23776-2018 (GB/T: National Standards of the People’s Republic of China) as follows: the samples (100–200 g) were placed in a tea evaluation tray, and their appearance was assessed from all angles. Next, 5 g of the sample was placed in a standard evaluation cup. Boiling water (110 mL) was added to the sample. After 1 minute of steeping, the lid was opened for aroma evaluation. After 2 minutes of steeping, the tea liquid was poured into the evaluation bowl for flavor and aroma evaluation. The process was repeated twice, with steeping durations of 3 and 5 minutes (Fig. 1, Supplementary Table 1). The aroma, liquor color, and taste of the tea infusions from the three brews mentioned above were evaluated, following which the results were subsequently scored. Sensory evaluations were conducted using the descriptive terms and scoring criteria in accordance with GB/T 14487-2017 and GB/T 21824-2008.
QDA was further employed to quantify the sensory quality of the YCFS samples (Supplementary Tables 2 and 3). Evaluators from Fujian Agriculture and Forestry University conducted role tests to evaluate their capacity for flavor differentiation, evaluation, description, and perception of tea, especially in YCFS flavor profiles. The evaluators were selected with an accuracy of 80% or more. National regulations do not require ethical approval or the establishment of human ethics committees for sensory review. Ultimately, 11 evaluators (6 females, 5 males, aged 22-50 years) were selected. Each evaluator was proficient and accurate in evaluating the test samples using descriptors and a 0-7 intensity scale (0 = none, 7 = extreme). They recorded the flavor and aroma descriptors for each sample. Twelve descriptors including thickness, sweetness, astringency, mellowness, sweetness, astringency, floral, fruity, sweet, woody, herbal, and green were ultimately selected to define the flavor of the YCFSs, and all of which had a usage rate of 80% or more.
Determination of the main macro-compounds
The content of tea polyphenol was determined using the Folin-Ciocalteu colorimetric method according to GB/T 8313-2018. Specifically, 0.2 g of the sample was added to 4.5 mL of 70% methanol (preheated to 70 °C). After stirring and cooling, the sample was centrifuged at 3500 r/min for 10 minutes, and the supernatant was transferred to a 10 mL test tube. Then, 1 mL of the supernatant was taken, added with 5 mL of a Folin-Ciocalteu reagent and 4 mL of 7.5% Na2CO3, shaken well for 8 minutes, added with pure water to reach the desired volume. The mixture was allowed to stand in the dark at room temperature for 60 min, and then the absorbance was measured at 765 nm. The tea polyphenol content was calculated and expressed as a percentage (%) based on gallic acid equivalent on a dry weight basis. The aluminum trichloride-sodium nitrite colorimetric method was employed to determine the total flavonoid content52. The colorimetric method according to GB/T 8314-2013 was used to determine the total free amino acid content. The results were expressed as a percentage (%) on a dry weight basis. Briefly, 1 mL of tea infusion and 0.5 mL of phosphate-buffered saline were put into 25 mL test tubes and then added with 0.5 mL of ninhydrin. The test tubes were placed in a boiling water bath for 15 min, cooled and then diluted with water to 25 mL. After being left to stand for 10 min, the absorbance of the samples at 570 nm was spectrophotometrically determined. Soluble sugars were determined using a plant-soluble sugar determination kit (Grace Biotechnology Co., Ltd, Suzhou, China) according to the manufacturer’s protocols, and the absorbance of the samples was measured at 620 nm. The water extract content, which represents the mass fraction of water-soluble substances extracted from the tea under specified conditions, was determined according to GB/T 8305-2013. Briefly, 3 g of tea samples were brewed with 450 mL of boiling water. A 50 mL aliquot of the tea infusion was then pipetted into an evaporating dish, evaporated to dryness, oven-dried at 120 °C for 1 h, cooled in a desiccator, and weighed. The result was calculated and expressed as a percentage (%) on a dry matter basis. The quantification of key oxidative tea pigments, namely theaflavins (TFs), thearubigins (TRs), and theabrownins (TBs), was conducted following the UV-vis spectrophotometric protocol established by Zhao et al.53. A series of organic solvents were systematically used to extract and separate tea leaves according to the referenced method, with absorbance values measured at a wavelength of 380 nm. The content of each pigment was calculated according to its respective formula and reported as a percentage (%) of dry mass.
Determination of the nonvolatile compounds by HPLC
The contents of caffeine and catechins (C, EC, GC, EGC, ECG, and EGCG) was measured using a Waters 2695 high performance liquid chromatograph (HPLC) system (Waters, Milford, MA, USA). The extraction was performed using a previously described method with slight modifications54. The tea powder sample (200 mg) was extracted with 5 mL of 70% (v/v) methanol at 70 °C for 10 min with intermittent shaking every 5 min. After centrifugation at 3500 r/min for 10 min, the supernatant was collected. The residue was re-extracted twice more using the same procedure (5 mL of 70% methanol, 70 °C, 10 min). The supernatant (1 mL) was filtered through a 0.45 μm nylon membrane and stored at −20 °C for HPLC analysis. The chromatographic conditions were as follows: 2998 PDA detector (Waters, Milford, MA, USA); X-select-T3 C18 column (4.6 mm × 250 mm, 5 µm; Waters, Milford, MA, USA) and maintained at 35 °C. Mobile phase A: 100% pure water +0.09% acetonitrile + 0.02% EDTA-2Na + 2% glacial acetic acid; mobile phase B: 80% acetonitrile. The following gradient elution program was applied at a constant flow rate of 1.0 mL/min: 0–11.5 min, 100% A; 11.5–15.5 min, 100–70% A; 15.5–24 min, 70% A; 24–30 min, 70–100% A; 30–35 min, 100% A. The PDA detection conditions were as follows: scanning range 200-400 nm, characteristic detection wavelength of 278 nm, the total run time was 35 min, and injection volume of 1 μl. All samples were subjected to three technical repetitions. The following standard stock solutions were prepared: 2.00 mg/mL caffeine, 1.00 mg/mL C, 1.00 mg/mL EC, 2.00 mg/mL GC, 2.00 mg/mL EGC, 2.00 mg/mL ECG, and 2.00 mg/mL EGCG. Quantification was performed using the external standard method. Calibration curves were constructed by plotting the peak areas against the concentrations of the standard solutions. The linear range was 5–200 µg/mL, with a correlation coefficient (R²) greater than 0.999. All samples were analyzed in triplicate, and the average values were used for data analysis.
Determination of aroma compounds via HS-SPME-GC–MS
Headspace solid phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS, QP2020NX Shimadzu, Kyoto, Japan) was employed to identify the aroma compounds. Briefly, 0.1 g of ground sample was weighed and placed into a 20 mL headspace vial and then added with 10 μL of an internal standard solution (10 ppm, ethyl decanoate, CAS 110-38-3) was added. The vials were sealed using crimp-top caps with TFE-silicone headspace septa (Agilent, Palo Alto, CA, USA) and were allowed to equilibrate at 60 °C for 10 minutes. Using a headspace solid-phase microextraction (HS-SPME) handheld device (57357U, Supelco) and a 70 µm carbon/polydimethylsiloxane (CAR/PDMS) fibre (Supelco, Bellefonte, PA, USA), which was conditioned prior to the first use by heating in the GC injection port at 270 °C for 1 h under a helium flow of 1.0 mL/min, the aged extraction heads were inserted into vials through the SPME handheld device that absorbed volatile compounds at 70 °C for 40 min. The fibers were inserted into a gas chromatograph sampler, and thermal desorption was performed at 250 °C for 5 min. Five biological replicates and three technical replicates were performed. The GC condition was as follows: the chromatographic column was SH-Rtx-5 (30 m × 0.25 mm, 0.25 µm, Shimadzu, Kyoto, Japan); carrier gas: helium (purity > 99.99%). Sampling was performed using at a split flow with a split flow ratio of 5:1. The heating procedure was as follows: the oven temperature was set at 50 °C; 4 °C/min to 100 °C for 3 min; 3 °C/min to 120 °C for 2 min; 2 °C/min to 150 °C for 2 minutes; 3 °C/min to 180 °C for 2 minutes, then increased to 230 °C at 5 °C/min. The MS was operated in electron excitation ionization mode with an electron energy of 70 eV and a scanning range of 30–550 m/z. Normal alkanes C7–C40 (Sigma-Aldrich Corporation, Saint Louis, MO, USA) were used as an external reference to calculate the retention indices (RIs) of each compound. Qualitative analyses were conducted using RI, built-in National Institute of Standards and Technology (NIST20) database.
The semi-quantitative analyses were conducted using internal standard method. The relative concentration of each analyse was derived from its GC-MS peak area ratio to the internal standard (ethyl decanoate). The relative concentration (RCvoc) of each aroma compound was calculated using Eq. (1).
RCvoc is the relative concentration (μg·g−1) of the aroma compound, Avoc is the chromatographic peak area, Asta is the internal standard peak area, mstq is the mass of the internal standard, and ms is the mass (g) of the samples.
Quantification of aroma compounds and calculation of OAVs
Aroma compounds were quantified using the peak areas of the internal standard compound. The OAV was calculated based on the ratio of the concentration of the key aroma compounds to their respective threshold values in water. The OAV of each component was calculated using Eq. (2).
Ci is the concentration(μg·kg−1); OTi (μg/L) is the odor threshold value of each compound in water.
Correlation analyses and construction of the flavor wheel
QDA led to the identification of 12 sensory descriptors for the YCFS that listed in the Supplementary Table 3. Correlation analyses were conducted between these flavor compounds as well as six taste and six aroma attributes. 13 out of 15 phytochemical components (p < 0.05, and correlation coefficient |r | > 0.6) were selected as key flavor compounds for correlation network. Using VIP > 1, p < 0.05, |r | > 0.6, and OAV > 1 as criteria55,56, 12 aroma compounds were identified as key flavor compounds for correlation network. Pearson correlation analysis was performed using Origin 2021 software, with visualization conducted in Cytoscape 9.1.
The QDA identified 12 sensory descriptors for the YCFS that component the second tier of the flavor wheel. Twelve phytochemicals (VIP > 1) were selected as key flavor substances for taste. Using VIP > 1, OAV > 1, and |r | > 0.6 as criteria, 10 volatile compounds were identified as key aroma substances. Correlation analyses were conducted between these key flavour substances and six taste and six aroma attributes, and the results were used to construct the content of the third layer of the YCFS flavour wheel.
Statistical analysis
Each of the four grades of the YCFS-S (SA, SB, SC, and SD) and YCFS-A samples (AA, AB, AC, and AD) samples consisted of five biological replicates, all of which were subjected to three technical replicates. All statistical analyses were performed using IBM SPSS Statistics 21 (SPSS Inc., Chicago). The final results were expressed as the mean ± standard deviation (n = 5) of all the replicate measurements and were used for subsequent analyses. The effects of season and tea grade on the contents of phytochemical and aroma compounds were determined by two-way analysis of variance (two-way ANOVA). For compounds that exhibited a significant season and grade interaction (p < 0.05), a simple effects analysis was conducted to examine the effect of tea grade within each fixed season, and vice versa. Bonferroni’s post-hoc test was subsequently applied for multiple comparisons within the simple effects to control the family-wise error rate at p < 0.05. Significant differences among groups are indicated by different lowercase letters in the tables. For compounds where the interaction effect was not significant (p > 0.05), the main effects of season and grade were interpreted separately. The main effect of season was analyzed using an independent samples t-test. The main effect of grade was analyzed by one-way ANOVA followed by Tukey’s HSD post-hoc test for multiple comparisons among the four grades. OPLS-DA (Orthogonal Partial Least Squares Discriminant Analysis) and PLS-DA (Partial Least Squares Discriminant Analysis) were conducted using MetWare Cloud Platform (https://cloud.metware.cn/). The significantly differentially metabolites were determined based on VIP > 1 and p < 0.05 obtained by the OPLS-DA/PLS-DA.
Data availability
Data will be made available on request.
References
Dubey, K. K., Janve, M., Ray, A. & Singhal, R. S. In Trends in Non-alcoholic Beverages (ed. C. M. Galanakis) 101–140 (Academic Press, 2020).
Tan, J. et al. Improving flavor of Wuyi rock tea processed from rain-soaked leaves by optimizing withering conditions. Food Chem 471, 142762 (2025).
Xu, K. et al. Non-targeted metabolomics analysis revealed the characteristic non-volatile and volatile metabolites in the Rougui Wuyi Rock Tea (Camellia sinensis) from different culturing regions. Foods 11, 1694 (2022).
Ma, B. et al. Impact of harvest season on bioactive compounds, amino acids and in vitro antioxidant capacity of white tea through multivariate statistical analysis. LWT 164, 113655 (2022).
Zhang, C. et al. A comprehensive investigation of macro-composition and volatile compounds in spring-picked and autumn-picked white tea. Foods 11, 3628 (2022).
Luo, Q., Luo, L., Zhao, J., Wang, Y. & Luo, H. Biological potential and mechanisms of tea’s bioactive compounds: An updated review. J. Adv. Res. 65, 345–363 (2024).
Chen, L. et al. Seasonal variation in non-volatile flavor substances of fresh tea leaves (Camellia sinensis) by integrated lipidomics and metabolomics using UHPLC-Q-Exactive mass spectrometry. Food Chem 462, 140986 (2025).
Zhou, P. et al. A multi-element stable isotopes approach coupled with chemometrics for the determination of Tieguanyin tea geographical origin and harvest season. Analytical Methods 11, 346–352 (2018).
Zhou, J. et al. Seasonal variations and sensory profiles of oolong tea: Insights from metabolic analysis of Tieguanyin cultivar. Food Chem 462, 140977 (2025).
Ye, J. et al. Taste characteristics and symbolic metabolites of Rougui tea with different grades in China. Food Chem.: X 27, 102405 (2025).
Zhu, M., Li, E. & He, H. Determination of volatile chemical constitutes in tea by simultaneous distillation extraction, vacuum hydrodistillation and thermal desorption. Chromatographia 68, 603–610 (2008).
Peng, J. et al. Auxiliary protective effect of Yongchun Foshou tea on ulcerative colitis in mice and analysis of major components involved. Food Sci 43, 129–136 (2022).
Li, W. et al. Composition characteristics of amino acids in Chinese teas based on large-scale samples: Origin, tea category, and harvest season. Food Res. Int. 222, 117616 (2025).
Deng, S. et al. Bitter and astringent substances in green tea: composition, human perception mechanisms, evaluation methods and factors influencing their formation. Food Res. Int. 157, 111262 (2022).
Koch, W. In Handbook of Dietary Phytochemicals. (eds. J. Xiao, S. D. Sarker & Y. Asakawa) 1-29 (Springer, Singapore 2020).
Wu, W. et al. Metabonomics analysis of the flavor characteristics of Wuyi Rock Tea (Rougui) with “rock flavor” and microbial contributions to the flavor. Food Chem 450, 139376 (2024).
Wang, W. et al. Effects of key components on the antioxidant activity of black tea. Foods 12, 3134 (2023).
Wang, L., Lin, Q., Song, Z. & Chen, L. Spectrophotometric determination of total flavonoids in tea. Acta Tea Sinica 62, 1–6 (2021).
Peng, J. et al. Study on the Chemical Constituents of Yongchun Foshou Oolong Tea Based on Metabolomics. Sci. Agricultura Sin. 55, 769–784 (2022).
Pires, M. A., Pastrana, L. M., Fuciños, P., Abreu, C. S. & Oliveira, S. M. Sensorial perception of astringency: oral mechanisms and current analysis methods. Foods 9, 1124 (2020).
Wei, J. et al. Biosynthesis and the transcriptional regulation of terpenoids in tea plants (Camellia sinensis). Int. J. Mol. Sci. 24, 6937 (2023).
Liu, Z., Chen, F., Sun, J. & Ni, L. Dynamic changes of volatile and phenolic components during the whole manufacturing process of Wuyi Rock tea (Rougui). Food Chem 367, 130624 (2022).
Liu, J. et al. Metabolomic analyses reveal distinct change of metabolites and quality of green tea during the short duration of a single spring season. J. Agric. Food Chem. 64, 3302–3309 (2016).
Zhang, S. et al. Metabolomics analysis reveals the patterns and mechanisms of volatile metabolite in oolong tea during storage. LWT 185, 115173 (2023).
Zeng, L. et al. Comparative analysis of different grades of Tieguanyin oolong tea based on metabolomics and sensory evaluation. LWT 174, 114423 (2023).
Pang, X. et al. Research on taste and aroma characteristics of dahongpao tea with different grades. Chemosensors 13, 134 (2025).
Deng, G. et al. Mechanism of key volatiles in osmanthus scented green tea on the improvement of its aroma and taste quality. Food Chem 460, 140560 (2024).
Zhou, Y. et al. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem 231, 78–86 (2017).
Zheng, C. et al. The impact of harvest season on oolong tea aroma profile and quality. Plants 14, 2378 (2025).
Baldermann, S. et al. Discrimination of green, oolong, and black teas by GC-MS analysis of characteristic volatile flavor compounds. Am. J. Anal. Chem. 05, 13 (2014).
Yang, Z., Baldermann, S. & Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 53, 585–599 (2013).
Wu, Y.-J., Kuan, Y.-C. & Sheu, F. Revealing the roles of solar withering and shaking processes on oolong tea manufacturing from transcriptome and volatile profile analysis. Food Res. Int. 201, 115586 (2025).
Yue, C., Wang, Z. & Yang, P. Review: the effect of light on the key pigment compounds of photosensitive etiolated tea plant. Bot. Stud. 62, 21 (2021).
Hamilton, E. I. Compilation of odour threshold values in air and water: edited by L. J. van Gemert and A. H. Nettenbreijer, National Institute for Water Supply, Voorburg, The Netherlands, 1977. Price: DFl. 22.00. Sci. Total Environ. 9, 300–301 (1978).
Liao, X. et al. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ‘Zhonghuang 1. Food Res. Int. 136, 109355 (2020).
Aloum, L., Alefishat, E., Adem, A. & Petroianu, G. Ionone is more than a violet’s fragrance: A review. Molecules 25, 5822 (2020).
Liu, C. et al. (R)-Linalool is a key indicator of aroma quality levels of a distinctive black tea (Camellia sinensis var. Yinghong No. 9). Ind. Crops Products 225, 120506 (2025).
Huang, F.-F. et al. Lipids: A noteworthy role in better tea quality. Food Chem 431, 137071 (2024).
Spencer, M., Sage, E., Velez, M. & Guinard, J.-X. Using single free sorting and multivariate exploratory methods to design a new coffee taster’s flavor wheel. J. Food Sci. 81, S2997–S3005 (2016).
Zhang, Y., Liu, X. & Lu, C. Study on primitive morpheme in sensory terminology and flavor wheel construction of chinese tea. J. Tea Sci. 39, 474–483 (2019).
Lin, Y. et al. Uncovering the Shuixian tea grades hierarchy in Chinese national standard: From sensory evaluation to microstructure and volatile compounds analysis. Food Chem 459, 140342 (2024).
Wang, Z., Gan, S., Sun, W. & Chen, Z. Quality characteristics of oolong tea products in different regions and the contribution of thirteen phytochemical components to its taste. Horticulturae 8, 278 (2022).
Ye, J.-H. et al. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol 123, 130–143 (2022).
Fu, Z. et al. Analysis of differences in the accumulation of tea compounds under various processing techniques, geographical origins, and harvesting seasons. Food Chem 430, 137000 (2024).
Liu, X. et al. GOLDEN 2-LIKE transcription factors regulate chlorophyll biosynthesis and flavonoid accumulation in response to UV-B in tea plants. Horticultural Plant J 9, 1055–1066 (2023).
Kotik, M., Kulik, N. & Valentová, K. Flavonoids as aglycones in retaining glycosidase-catalyzed reactions: prospects for green chemistry. J. Agric. Food Chem. 71, 14890–14910 (2023).
Zhang, P. et al. Revealing the characteristic honey-like aroma formation in honey-scented black tea via molecular sensory analysis. Food Chem 493, 146023 (2025).
He, C. et al. The aroma characteristics of oolong tea are jointly determined by processing mode and tea cultivars. Food Chem.: X 18, 100730 (2023).
Zeng, L. et al. Uncovering reasons for differential accumulation of linalool in tea cultivars with different leaf area. Food Chem 345, 128752 (2021).
Hidalgo, F. J. & Zamora, R. Formation of phenylacetic acid and benzaldehyde by degradation of phenylalanine in the presence of lipid hydroperoxides: New routes in the amino acid degradation pathways initiated by lipid oxidation products. Food Chem. X 2, 100037 (2019).
Yin, P. et al. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol 129, 221–232 (2022).
Marinova, D., Ribarova, F. & Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 40, 255–260 (2005).
Zhao, F. et al. Macro-composition quantification combined with metabolomics analysis uncovered key dynamic chemical changes of aging white tea. Food Chem 366, 130593 (2022).
Xiang, P. et al. Light control of catechin accumulation is mediated by photosynthetic capacity in tea plant (Camellia sinensis). BMC Plant Biol 21, 478 (2021).
Gao, Y.-F., Zhou, Z.-X., Wang, M.-J. & Zhang, Y.-H. Volatile markers for unifloral safflower honey: An untargeted and targeted metabolomics analysis. Food Chem 471, 142734 (2025).
Qi, H., Ding, S., Pan, Z., Li, X. & Fu, F. Characteristic volatile fingerprints and odor activity values in different citrus-tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 25, 6027 (2020).
Zhu, J. et al. Comparison of aroma-active volatiles in oolong tea infusions using GC–Olfactometry, GC–FPD, and GC–MS. J. Agric. Food Chem. 63, 7499–7510 (2015).
Acknowledgements
This research was funded by the Young Backbone Faculty Fostering Fund of Anxi Tea College, Fujian Agriculture and Forestry University (ACKY2024002), the Establishment of Key Technology System for High Quality of Yongchun Foshou Oolong Tea (KH240283A), the Industry Development Plan of the Yongchun Foshou Oolong Tea of Sukeng Township (KH230073A), and the Special Fund for Science and Technology Innovation of Fujian Zhang Tianfu Tea Development Foundation (FJZTF01).
Author information
Authors and Affiliations
Contributions
Anru Zheng: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Writing – original draft, Visualization. Minghua Dong: Conceptualization, Validation. Zhendong Zhang: Visualization, Investigation, Software. Jiaxin Fang: Formal analysis, Software. Yu Pan: Visualization, Investigation, Data curation. Lele Jiang: Formal analysis, Validation. Caiyun Tian: Formal analysis, Validation. Jiayao Tan: Methodology, Data curation. Siwei Deng: Data curation. Cheng Zhang: Validation. Zhong Wang: Methodology. Chengzhe Zhou: Conceptualization, Validation, Writing – original draft, Supervision. Yuqiong Guo: Conceptualization, Funding acquisition, Project administration, Resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, A., Dong, M., Zhang, Z. et al. Key compounds responsible for the flavor diversity of Yongchun Foshou oolong tea of different grades and in different seasons. npj Sci Food 10, 13 (2026). https://doi.org/10.1038/s41538-025-00663-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00663-w