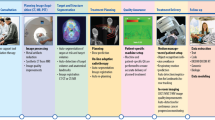
Abstract
This perspective article discusses emerging advances at the interface of mechanistic modeling and data-driven machine learning, highlighting opportunities for AI to accelerate discovery, improve predictive modeling, and enhance clinical decision-making. We address critical limitations of current AI approaches and propose a perspective on a future where AI augments mechanistic rigor, clinical relevance, and human creativity under the umbrella of a redefined understanding of Mathematical Oncology.
Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Ren, F. et al. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat. Biotechnol. 43, 63–75 (2025).
Podina, L., Ghodsi, A. & Kohandel, M. Learning chemotherapy drug action via Universal Physics-Informed Neural Networks. Pharm. Res. 42, 593–612 (2025).
Brummer, A. B. et al. Data driven model discovery and interpretation for CAR T-cell killing using sparse identification and latent variables. Front. Immunol. 14, 1115536 (2023).
Flaschel, M., Kumar, S. & De Lorenzis, L. Unsupervised discovery of interpretable hyperelastic constitutive laws. Comput. Methods Appl. Mech. Eng. 381, 113852 (2021).
Linka, K. & Kuhl, E. Best-in-class modeling: a novel strategy to discover constitutive models for soft matter systems. Extrem. Mech. Lett. 70, 102181 (2024).
Krenn, M., Kottmann, J. S., Tischler, N. & Aspuru-Guzik, A. Conceptual understanding through efficient automated design of quantum optical experiments. Phys. Rev. X. 11 (2021).
Trinh, T. H., Wu, Y., Le, Q. V., He, H. & Luong, T. Solving olympiad geometry without human demonstrations. Nature 625, 476–482 (2024).
Yang, K. et al. Position: Formal Mathematical Reasoning—A New Frontier in AI, (2025).
de Moura, L., Kong, S., Avigad, J., van Doorn, F. & von Raumer, J. The lean theorem prover (system description). in Automated Deduction—CADE-25 378–388 (Springer International Publishing, 2015). https://doi.org/10.1007/978-3-319-21401-6_26.
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Klemm, F. et al. MSK-ACCESS powered with SOPHiA DDM: Performance analysis of a decentralized MSK-ACCESS solution. J. Clin. Oncol. 42, e15063–e15063 (2024).
Pozzorini, C. et al. GIInger predicts homologous recombination deficiency and patient response to PARPi treatment from shallow genomic profiles. Cell Rep. Med. 4, 101344 (2023).
Zhao, C. et al. Is chain-of-Thought reasoning of LLMs a mirage? A data distribution lens. arXiv [cs.AI] (2025).
Swanson, K., Wu, W., Bulaong, N. L., Pak, J. E. & Zou, J. The Virtual Lab of AI agents designs new SARS-CoV-2 nanobodies. Nat. 1–8 https://doi.org/10.1038/s41586-025-09442-9 (2025).
Yamada, Y. et al. The AI Scientist-v2: Workshop-level automated scientific discovery via agentic tree search. arXiv [cs.AI] https://doi.org/10.48550/ARXIV.2504.08066 (2025).
McCall, A. & Mccall, A. AI for scientific discovery: Automating hypothesis generation. Authorea Inc. https://doi.org/10.22541/au.175044376.61922933/v1 (2025).
Alkan, A. K. et al. A survey on hypothesis generation for scientific discovery in the era of Large Language Models. arXiv [cs.CL] https://doi.org/10.48550/ARXIV.2504.05496 (2025).
Schneider, J. Generative to Agentic AI: survey, conceptualization, and challenges. arXiv [cs. AI] https://doi.org/10.48550/ARXIV.2504.18875 (2025).
Zhang, R. Z. et al. Personalized predictions of glioblastoma infiltration: mathematical models, physics-informed neural networks and multimodal scans. Med. Image Anal. 101, 103423 (2025).
Christenson, C. et al. Personalizing neoadjuvant chemotherapy regimens for triple-negative breast cancer using a biology-based digital twin. NPJ Syst. Biol. Appl. 11, 53 (2025).
Camacho-Gomez, D. et al. Physics-informed machine learning digital twin for reconstructing prostate cancer tumor growth via PSA tests. NPJ Digit. Med. 8, 485 (2025).
Lorenzo, G. et al. A pilot study on patient-specific computational forecasting of prostate cancer growth during active surveillance using an imaging-informed biomechanistic model. Cancer Res. Commun. 4, 617–633 (2024).
Lu, J., Deng, K., Zhang, X., Liu, G. & Guan, Y. Neural-ODE for pharmacokinetics modeling and its advantage to alternative machine learning models in predicting new dosing regimens. iScience 24, 102804 (2021).
Metzcar, J., Jutzeler, C. R., Macklin, P., Köhn-Luque, A. & Brüningk, S. C. A review of mechanistic learning in mathematical oncology. Front. Immunol. 15, 1363144 (2024).
Zhao, A., Fattahi, D. & Hu, X. Physics-informed neural networks for physiological signal processing and modeling: a narrative review. Physiol. Meas. 46, 07TR02 (2025).
Laurie, M. & Lu, J. Explainable deep learning for tumor dynamic modeling and overall survival prediction using Neural-ODE. NPJ Syst. Biol. Appl. 9, 58 (2023).
Hu, Z., Daryakenari, N. A., Shen, Q., Kawaguchi, K. & Karniadakis, G. E. State-space models are accurate and efficient neural operators for dynamical systems. arXiv [cs.LG] (2024).
Mao, R., Wan, L., Zhou, M. & Li, D. Cox-Sage: enhancing Cox proportional hazards model with interpretable graph neural networks for cancer prognosis. Brief. Bioinform. 26 (2025).
Bigarré, C. et al. Mechanistic modeling of metastatic relapse in early breast cancer to investigate the biological impact of prognostic biomarkers. Comput. Methods Prog. Biomed. 231, 107401 (2023).
Gallagher, K. et al. Mathematical model-driven deep learning enables personalized adaptive therapy. Cancer Res 84, 1929–1941 (2024).
Hammond, J. & Smith, V. A. Bayesian networks for network inference in biology. J. R. Soc. Interface 22, 20240893 (2025).
Canonaco, F., Gaudillo, J., Astrologo, N., Stella, F. & Acerbi, E. A guide to Bayesian networks software for structure and parameter learning, with a focus on causal discovery tools. Front. Syst. Biol. 5, 1631901 (2025).
Gogoshin, G. & Rodin, A. S. Minimum uncertainty as Bayesian network model selection principle. BMC Bioinforma. 26, 100 (2025).
Zhang, M.-N. et al. Comprehensive review of Bayesian network applications in gastrointestinal cancers. World J. Clin. Oncol. 16, 104299 (2025).
Hong, T., Xie, S., Liu, X., Wu, J. & Chen, G. Do machine learning approaches perform better than regression models in mapping studies? A systematic review. Value Health 28, 800–811 (2025).
Jiang, P. et al. Big data in basic and translational cancer research. Nat. Rev. Cancer 22, 625–639 (2022).
Kazerouni, A. S. et al. Integrating quantitative assays with biologically based mathematical modeling for predictive oncology. iScience 23, 101807 (2020).
Bi, W. L. et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J. Clin. 69, 127–157 (2019).
Wu, C. et al. A critical assessment of artificial intelligence in magnetic resonance imaging of cancer. Npj Imaging 3, 15 (2025).
Lipkova, J. et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 40, 1095–1110 (2022).
Lorenzo, G. et al. Patient-specific, mechanistic models of tumor growth incorporating artificial intelligence and big data. Annu. Rev. Biomed. Eng. 26, 529–560 (2024).
Metzcar, J., Wang, Y., Heiland, R. & Macklin, P. A review of cell-based computational modeling in cancer biology. JCO Clin. Cancer Inform. 3, 1–13 (2019).
Hernandez-boussard, T. et al. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat. Med. https://doi.org/10.1038/s41591-021-01558-5 (2021).
Abdollahi, H. et al. Theranostic digital twins: Concept, framework and roadmap towards personalized radiopharmaceutical therapies. Theranostics 14, 3404–3422 (2024).
Fung, L., Fasel, U. & Juniper, M. Rapid Bayesian identification of sparse nonlinear dynamics from scarce and noisy data. Proc. Math. Phys. Eng. Sci. 481 (2025).
Bastek, J.-H., Sun, W. & Kochmann, D. M. Physics-informed diffusion models. arXiv [cs.LG] (2024).
Laslo, D. et al. Mechanistic learning with guided diffusion models to predict spatio-temporal brain tumor growth. arXiv [cs.CV] https://doi.org/10.48550/ARXIV.2509.09610 (2025).
Ciccolini, J., Barbolosi, D., André, N., Barlesi, F. & Benzekry, S. Mechanistic learning for combinatorial strategies with immuno-oncology drugs: can model-informed designs help investigators? J. Clin. Oncol. Precision Med. 486–491 https://doi.org/10.1200/PO.19.00381 (2020).
Velten, B. et al. Identifying temporal and spatial patterns of variation from multimodal data using MEFISTO. Nat. Methods 19, 179–186 (2022).
Zhu, J., Sun, S. & Zhou, X. SPARK-X: non-parametric modeling enables scalable and robust detection of spatial expression patterns for large spatial transcriptomic studies. Genome Biol. 22, 184 (2021).
Zhang, K., Feng, W. & Wang, P. Identification of spatially variable genes with graph cuts. Nat. Commun. 13, 5488 (2022).
Liu, N. et al. standR: spatial transcriptomic analysis for GeoMx DSP data. Nucleic Acids Res. 52, e2 (2024).
Peng, X. et al. Scalable topic modelling decodes spatial tissue architecture for large-scale multiplexed imaging analysis. Nat. Commun. 16, 6619 (2025).
Arango-Argoty, G. et al. AI-driven predictive biomarker discovery with contrastive learning to improve clinical trial outcomes. Cancer Cell 43, 875–890.e8 (2025).
Swanson, K., Wu, E., Zhang, A., Alizadeh, A. A. & Zou, J. From patterns to patients: Advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell 186, 1772–1791 (2023).
Scott, J. M., Stene, G., Edvardsen, E. & Jones, L. W. Performance status in cancer: not broken, but time for an upgrade?. J. Clin. Oncol. 38, 2824–2829 (2020).
Pati, S. et al. Federated learning enables big data for rare cancer boundary detection. Nat. Commun. 13, 7346 (2022).
Zhu, M. et al. AI Scientists fail without strong implementation capability. arXiv [cs.AI] https://doi.org/10.48550/ARXIV.2506.01372 (2025).
Hu, X. et al. Nova: An iterative planning and search approach to enhance novelty and diversity of LLM generated ideas. arXiv [cs.AI] https://doi.org/10.48550/ARXIV.2410.14255 (2024).
Padigela, H., Shah, C. & Juyal, D. ML-Dev-Bench: Comparative Analysis of AI Agents on ML development workflows. arXiv [cs.SE] https://doi.org/10.48550/ARXIV.2502.00964 (2025).
Mennella, C., Maniscalco, U., De Pietro, G. & Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: a narrative review. Heliyon 10, e26297 (2024).
Farhud, D. D. & Zokaei, S. Ethical issues of artificial intelligence in medicine and healthcare. Iran. J. Public Health 50, i–v (2021).
Dankwa-Mullan, I. Health equity and ethical considerations in using artificial intelligence in public health and medicine. Prev. Chronic Dis. 21, E64 (2024).
Khan, U. S. & Khan, S. U. R. Ethics by design: A lifecycle framework for trustworthy AI in medical imaging from transparent data governance to clinically validated deployment. arXiv [cs. CY] https://doi.org/10.48550/ARXIV.2507.04249 (2025).
Hassan, M., Borycki, E. M. & Kushniruk, A. W. Artificial intelligence governance framework for healthcare. Healthc. Manag. Forum 38, 125–130 (2025).
Jobin, A., Ienca, M. & Vayena, E. The global landscape of AI ethics guidelines. Nat. Mach. Intell. 1, 389–399 (2019).
Spiegelhalter, D. Why probability probably doesn’t exist (but it is useful to act like it does). Nature 636, 560–563 (2024).
Robeva, R. S., Jungck, J. R. & Gross, L. J. Changing the nature of quantitative biology education: Data science as a driver. Bull. Math. Biol. 82, 127 (2020).
Zha, S. et al. A case study of integrating AI literacy education in a biology class. Int. J. Artif. Intell. Educ. https://doi.org/10.1007/s40593-025-00476-8 (2025).
Larson, B. Z., Moser, C., Caza, A., Muehlfeld, K. & Colombo, L. A. Critical thinking in the age of generative AI. Acad. Manag. Learn. Educ. 23, 373–378 (2024).
Naqvi, W. M., Ganjoo, R., Rowe, M., Pashine, A. A. & Mishra, G. V. Critical thinking in the age of generative AI: implications for health sciences education. Front. Artif. Intell. 8, 1571527 (2025).
Papaneophytou, C. & Nicolaou, S. A. Promoting critical thinking in biological sciences in the era of artificial intelligence: The role of higher education. Trends High. Educ. 4, 24 (2025).
Kannan, M., Bridgewater, G., Zhang, M. & Blinov, M. L. Leveraging public AI tools to explore systems biology resources in mathematical modeling. NPJ Syst. Biol. Appl. 11, 15 (2025).
Rockne, R. et al. The Future of Mathematical Oncology in the Age of AI https://mathematical-oncology.org/blog/future-of-math-oncology.html (2025).
Acknowledgements
The authors acknowledge the generosity of the city of Ortigia, Syracuse, Sicily, for providing the meeting venue and refreshments and the Beckman Research Institute at City of Hope for financial support.
Author information
Authors and Affiliations
Contributions
R.C.R. attended the meeting in Syracuse and contributed to and approved the final manuscript. M.A. attended the meeting in Syracuse and contributed to and approved the final manuscript. A.R.A.A. attended the meeting in Syracuse and contributed to and approved the final manuscript. D.B. attended the meeting in Syracuse and contributed to and approved the final manuscript. A.B. attended the meeting in Syracuse and contributed to and approved the final manuscript. S.Be. attended the meeting in Syracuse and contributed to and approved the final manuscript. S.Br. attended the meeting in Syracuse and contributed to and approved the final manuscript. S.C.Bru. attended the meeting in Syracuse and contributed to and approved the final manuscript. M.C. attended the meeting in Syracuse and contributed to and approved the final manuscript. F.F. attended the meeting in Syracuse and contributed to and approved the final manuscript. A.K. attended the meeting in Syracuse and contributed to and approved the final manuscript. A.K.-L. attended the meeting in Syracuse and contributed to and approved the final manuscript. G.L. attended the meeting in Syracuse and contributed to and approved the final manuscript. B.M. attended the meeting in Syracuse and contributed to and approved the final manuscript. A.S.R. contributed to and approved the final manuscript. L.S. attended the meeting in Syracuse and contributed to and approved the final manuscript. J.S. attended the meeting in Syracuse and contributed to and approved the final manuscript. C.T. attended the meeting in Syracuse and contributed to and approved the final manuscript. K.U. attended the meeting in Syracuse and contributed to and approved the final manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rockne, R.C., Andersen, M., Anderson, A.R.A. et al. The future of mathematical oncology in the age of AI. npj Syst Biol Appl (2026). https://doi.org/10.1038/s41540-026-00656-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41540-026-00656-9