Abstract
Schistosomiasis is a neglected tropical disease with the greatest burden in sub-Saharan Africa. An efficacious and safe vaccine would have a major global public health impact. The investigational SchistoShield® (Sm-p80 [antigen] + GLA-SE [adjuvant]) vaccine targets the Sm-p80 surface membrane antigen of Schistosoma mansoni and in nonhuman primate challenge studies was shown to be highly effective in killing pathogenic female worms and reducing host organ pathology and egg excretion. In this Phase 1 first-in-human, dose-escalation trial with sequential assignment, we evaluated the safety and immunogenicity of the vaccine in healthy adults in the United States. The vaccine formulations, given as a three dose intramuscular series, were well tolerated and adjuvanted formulations induced robust IgG ELISA responses against the Sm-p80 antigen. The vaccine has been advanced to a Phase 1b trial among adults in endemic areas of Africa.
Clinicaltrials.gov registration: NCT05292391 https://Clinicaltrials.gov/study/NCT05292391.
Similar content being viewed by others
Schistosomiasis is a poverty-related neglected tropical disease, impacting more than 700 million people who live in endemic areas and are at risk of infection1. Chemotherapy with praziquantel is the current preferred method for schistosomiasis control; however, the effectiveness of mass-treatment programs is compromised by reinfection requiring regular re-treatment2. An efficacious vaccine with long-lasting protection against all schistosomiasis forms could have a major impact on this ancient disease3,4.
Here we report the first-in-human clinical trial of a potent schistosomiasis vaccine (SchistoShield®) which is based on a defined Schistosoma mansoni antigen, the large subunit of the calcium-activated neutral protease termed Sm-p80. Sm-p80 plays an important role in apical surface membrane biogenesis, a phenomenon widely believed to be an immune evasion process employed by the hemo-helminth schistosome parasite5. Effectiveness of SchistoShield® against both intestinal and hepatic disease has been extensively tested in animal models including mice, hamsters, and baboons6,7,8,9,10,11,12,13,14. In the non-human primate model, SchistoShield® was effective against all major schistosome species and, notably, is the only vaccine candidate to consistently exhibit potent prophylactic (kills infectious larvae), therapeutic (kills existing worms in the host), transmission-blocking (reduces egg viability and egg expulsion into the environment), and pathology reducing (decreases the quantity of eggs and granulomas in tissues) efficacy14.
These preclinical findings supported the initiation of this first-in-human, dose-escalation, Phase 1 study of SchistoShield® in healthy adults in a non-endemic area.
Results
Trial population
Of the 45 participants enrolled, 42 received all three vaccinations, including all participants in Groups A, B, and C. Two participants discontinued treatment due to an AE: one participant from Group D discontinued treatment due to worsening anxiety not related to vaccination and one participant from Group E discontinued treatment due to a mild vesicle at the injection site related to the study vaccination. Another participant in Group D was lost to follow up after the first vaccination (Fig. 1). Baseline characteristics were relatively similar across study groups, with some variation in the distribution by sex across the study groups (Table 1).
Safety
Overall, the vaccines were well tolerated. Of the 45 enrolled participants, 43 (96%) experienced at least one local solicited AE, 37 (82%) experienced at least one systemic solicited AE, and 39 (87%) experienced at least one unsolicited AE. All solicited systemic AEs were mild to moderate in severity (Table 2). Fatigue and headache were the most common solicited systemic AEs reported, occurring in 69% and 58% of participants, respectively, after any vaccination, and occurring in relatively similar proportions across study groups and following each of the three study vaccinations. Fever was reported by only one participant, in Group E, following the second vaccination, and was mild in severity. There were four reports of arthralgia, defined as a solicited event if there was generalized or multifocal joint pain, of mild severity across three participants, one of whom reported arthralgia after two vaccinations, all of whom were in Group A.
Solicited local AEs were nearly exclusively mild or moderate in severity. Only two local AEs were judged severe, including one report of the size of an area of erythema/redness (after the second vaccination in a Group C participant) and one report of tenderness (after the first vaccination in a Group E participant). The frequency of reported solicited local AEs was relatively consistent across study groups and following each of the three vaccinations, with tenderness (96%) and pain (65%) most commonly reported.
The most common related unsolicited AE within 28 days post vaccination was arthralgia, defined as an unsolicited event if there was pain in one joint, which was reported by three participants across Groups A and B. In Group A, two participants experienced mild arthralgia, one after dose 2 and another after dose 3. Although one of those participants had symptoms in two joints in the hand, the event was reported as an unsolicited AE as it was not judged to be generalized arthralgia. In Group B, one participant experienced mild arthralgia after dose 1.
Other unsolicited AEs judged related to vaccination included a single record of each of the following: lymphadenopathy, tachycardia, injection site vesicles, injection site warmth, face swelling, muscular weakness, plantar fasciitis, tendon disorder, dizziness, hypoesthesia, and cough. All were graded mild in severity, except for plantar fasciitis and injection site warmth, which were graded as moderate. Thirty-seven (82%) participants reported at least one unrelated unsolicited AE, with the most common of these events being upper respiratory tract infection (20%) and vessel puncture site hemorrhage (13%). One participant in Group D had grade 3 tachycardia judged unrelated to study product. One NOCMC, of thyroid nodules of mild severity judged unrelated to vaccination, was reported in a Group A participant. No SAEs, MAAEs, PIMMCs, or deaths were reported.
Clinical laboratory evaluations assessed before and after vaccinations included hemoglobin, white blood cells, platelets, alanine aminotransferase, and creatinine. Of the laboratory measurements defined as AEs related to vaccination, two participants had one measurement of mildly increased creatinine, two participants had one measurement of a mild decrease in white blood cells, two participants had more than one measurement of a mildly increased platelet count and one participant had one measurement of a mildly increased platelet count. Three female participants had mild or moderate decreased hemoglobin.
Immunogenicity
Baseline samples were tested in duplicate for anti-Sm-p80 IgG ELISA levels. Only three participants had baseline anti-Sm-p80 IgG ELISA values below the lower limit of quantification (LLOQ) (IgG end point titer of 1.000 on the log10 scale) for both read-outs, and another six participants had one value below and one above that level. Among the other participants, one (in Group C) had a relatively high baseline endpoint titer of 3715, one had a titer of 372, two others had baseline titers of 288 (one of whom was in Group C), and all other participants with duplicate values above the LLOQ had baseline values less than 170. All participants denied, as a condition of enrollment, having had known schistosomiasis infection or having traveled to an endemic area for schistosomiasis and been potentially exposed. Among the Groups, the highest nominal Day 1 baseline GMT, of 75, was observed in Group C but this value was not statistically significant compared to the baseline GMTs observed in the other groups (Table 3).
Seropositivity status at baseline did not appear to influence the magnitude of responses to the vaccine and responses were induced in both baseline seropositive and seronegative participants. For example, the participant with the baseline titer of 3715 responded with titers of 6166 and 10,233 at 28 days after the second and third vaccinations, respectively.
In an ad hoc sensitivity analysis excluding the Group C participant with the baseline titer of 3715, the Group C GMTs through Day 29 in the ad hoc analysis were somewhat lower than the Group C GMTs in the analyses of all participants (reported in Table 3), with a baseline GMT of 46 vs 75 and a Day 29 GMT of 95 vs 139, all with wide confidence intervals (CIs). After Day 29, there was little difference in the Group C GMTs reported in the ad hoc analysis and those reported in Table 3, for example, with Day 36 and 57 GMTs of 952 vs 1080 and 2427 vs 2692, respectively, suggesting that the outlier at baseline had little influence on the overall findings.
All formulations induced an increase in anti-Sm-p80 IgG ELISA GMTs, with consistently lower responses in the unadjuvanted Group A participants compared with the adjuvanted Groups (Table 3 and Fig. 2). However, there was no significant increase in anti-Sm-p80 IgG GMTs, as evidenced by the overlapping CIs compared to baseline, at 7 or 28 days after Dose 1 in any study group. At 28 days after Dose 2, there was a significant increase in GMT, compared with baseline, in all Groups, with the greatest increases in the adjuvanted Groups. Among those Groups, Group C (delayed booster) had the highest peak response after Dose 2, although there were wide CIs around those estimates.
In the analysis of all participants, increased GMTs were observed at 7 and 28 days following Dose 2 in all groups, and Group C had significantly greater GMT responses compared to Group A (unadjuvanted group), as evidenced by the non-overlapping CIs, at those time points. The pre-Dose 3 GMT at Day 180 for Group C (376) was numerically lower than the pre-Dose 3 GMTs at Day 57 in the other adjuvanted vaccine groups (869, 869, and 1660 for groups B, D, and E respectively). For all Groups, peak GMT responses were observed at 28 days after Dose 3, with the highest nominal response in Group C.
Among the Groups vaccinated on the Day 1, 29, and 57 schedule, the lowest nominal response at 28 days after Dose 3 was observed in Group A (GMT 1187), while responses in Groups B, D, and E were comparable (GMTs 4255, 4074, and 4480 respectively), suggesting a lack of a dose response across the Groups given 10, 30, or 100 µg Sm-p80 antigen plus adjuvant. Geometric mean titers subsequently declined in all Groups at the final time point of 124 days after Dose 3 (Groups A, B, D, and E [514, 1413, 440, and 986, respectively] and Group C [1472], but remained significantly higher than baseline, as evidenced by the non-overlapping CIs.
These results indicate that all vaccine formulations induced anti-Sm-p80 antibody responses and that responses were greater with the adjuvanted formulations. Since there were only 9 patients in each group, the confidence intervals were wide and, in general, overlapping, and there was no statistically significant difference in the antibody responses across the three study groups given varying dosages of Sm-p80 plus 5 μg GLA-SE adjuvant on a Day 1, 29, and 57 schedule. The delayed booster dose induced a nominally higher GMT at 28 days after Dose 3 compared with the other adjuvanted Groups but by the end of follow-up had declined to levels comparable to those observed in the other adjuvanted groups. Future studies with a higher number of participants may indicate a dose response relationship.
Discussion
We report the findings from this Phase 1, first-in-human, clinical trial of SchistoShield® vaccine formulations, with and without GLA-SE adjuvant. The study vaccine formulations were associated with acceptable safety profiles, and no SAEs, MAAEs, PIMMCs, or deaths were reported. Among the 45 participants enrolled in the study, local and systemic solicited AEs were commonly reported, by 96% and 82% of participants, respectively, with fatigue, headache, and myalgia the most commonly reported solicited systemic AEs. Tenderness was reported by all but two participants following any dose with one report judged as severe. Notably, fever was reported by only one participant, in Group E following the second vaccination.
Also of note, the four reports of the solicited systemic AE of arthralgia occurred exclusively in the unadjuvanted 100 µg dose Group A and the three reports of the related unsolicited AE of arthralgia included two participants in Group A and one in Group B. While it is possible that the clustering of these events in the unadjuvanted Group is due to chance, the occurrence of these events suggests the need to monitor such AEs in subsequent trials of the adjuvanted formulation of this vaccine. Two participants discontinued treatment due to an AE: one participant from Group D discontinued treatment due to moderate worsening anxiety not related to vaccination and one participant from Group E discontinued treatment due to a mild vesicle at the injection site judged related to the study vaccination.
All formulations induced anti-Sm-p80 IgG responses, with the lowest responses in the unadjuvanted Group A. Among the three adjuvanted formulations given on the Day 1, 29, and 57 schedule, the GMTs at 28 days after the third vaccination were comparable. The delayed booster vaccine schedule induced a nominally higher GMT at 28 days after the third vaccination, which declined over time to levels similar to those induced by the standard interval schedule.
Most participants had detectable levels of anti-Sm-p80 IgG responses at baseline and four had endpoint titers ≥170, including one participant with a baseline level of 3715. Another Phase 1 trial, of a recombinant S. mansoni vaccine targeting the tetraspanin 2 surface protein, in healthy adults in the United States, also found detectable serum levels of IgG against the target protein at baseline, in 7 of the 61 (11%) volunteers15. In that study, among the seropositive volunteers who received the study vaccine (instead of placebo), there was no appreciable response to the three-dose vaccine series. This is in contrast to our findings, indicating consistent increases in anti-Sm-p80 IgG responses post-vaccination among participants with detectable anti-Sm-p80 IgG antibodies at baseline. The baseline seropositivity among our non-endemic study population is likely due to cross-reactivity between Sm-p80 and other antigens. This has been shown, for example, with cross-reactivity of S. mansoni egg antigens with peanut and other plant allergens16. As previously noted15 it is also possible that prior exposure to other avian or mammalian schistosomes that cause cercarial dermatitis (swimmer’s itch) could induce cross reactive responses.
The results from this trial conducted in a non-endemic area supported the initiation in 2023 of a larger Phase 1b trial among schistosome-endemic populations in Africa (Madagascar and Burkina Faso) (NCT05762393), evaluating the 30 mcg adjuvanted SchistoShield® formulation given on the three-dose standard schedule, which is to be followed by a Phase 2 trial, also in Africa. In addition, SchistoShield® is concurrently being tested in a schistosome human challenge infection model in The Netherlands and in Uganda.
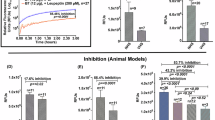
As the vaccine candidates advance to later-stage clinical trials it will be important to have one or more correlates of protection, in order to evaluate vaccine efficacy against this complex multicellular helminth schistosome parasite which employs an efficient immune evasion strategy and has a complex life cycle. To this end, progress has been made to develop a reproducible, quantitative, and functional assay in which schistosomal calpain (Sm-p80) inhibition by anti-Sm-p-80 antibodies induced by vaccination can be measured17. Significant inhibition by Sm-p80-specific antibodies produced by immunized mice, non-human primates, as well as participants in this clinical trial, was observed. These results suggest that inhibition of enzyme activity could serve as an important vaccine surrogate of protection in future Phase 3 trials.
The World Health Organization launched a neglected tropical diseases Road Map for 2021–2030 that targets the elimination of schistosomiasis in all endemic countries and calls for development of vaccines for humans, and animals, to prevent reinfection and reduce transmission. Currently, multiple human vaccine candidates targeting different schistosome antigens are in clinical trials3, including the recombinant S. mansoni Tetraspanin-2 Alhydrogel vaccine, evaluated in early phase trials funded by NIAID15,18, which is also being evaluated in a Phase 2 trial in Uganda (NCT03910972). Advances in technology and an increased focus on preventative methodologies have the potential to reduce the burden of this impactful disease. The results of this trial support further development of the Sm-p80 vaccine.
Methods
Trial design and participants
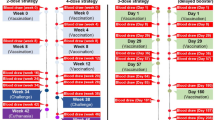
We conducted a Phase 1, first-in-human, open-label, dose-escalation trial designed to determine the safety, reactogenicity, and immunogenicity of three intramuscular (IM) injections of this recombinant Sm-p80 protein vaccine with and without adjuvant. Participants were sequentially assigned to Groups A through E (Table 1). Group A received a dose of 100 µg without adjuvant, Group B received a dose of 10 µg Sm-p80 with 5 µg of GLA-SE adjuvant, Groups C and D received a dose of 30 µg Sm-p80 with 5 µg of GLA-SE adjuvant, and Group E received a dose of 100 µg Sm-p80 with 5 µg of GLA-SE adjuvant. Groups A (unadjuvanted comparator), B, D, and E (low dose, mid dose, and high dose standard schedule, respectively) received vaccinations on Days 1, 29, and 57; Group C received vaccinations on Days 1, 29, and 180 (mid dose delayed schedule) (n = 9 per Group).
Eligible participants were males and non-pregnant females 19 through 55 years of age, in good health or with controlled chronic illness, without immunosuppression, and without known history of schistosomiasis. Complete inclusion and exclusion criteria are provided on clinicaltrials.gov (NCT05292391). Forty-five participants who provided written informed consent for study participation were enrolled at a single site in Seattle, WA between May 23, 2022 and January 24, 2023.
We evaluated safety and tolerability by identification of serious adverse events (SAEs) and medically-attended adverse events (MAAEs) (including new-onset chronic medical conditions [NOCMCs] and potentially immune-mediated medical conditions [PIMMCs]) from the time of the first study vaccination through 12 months after the last study vaccination; other unsolicited AEs from the time of each study vaccination through 28 days after each study vaccination; clinical safety laboratory AEs prior to and at 28 days after each study vaccination; and solicited local and systemic AEs, as reported on a daily study diary from the time of each study vaccination through 7 days after each vaccination. Solicited local (injection site) AEs included pruritus, erythema, induration/swelling, pain, and tenderness. Solicited systemic AEs included fever, chills, fatigue, malaise, myalgia, arthralgia, headache, nausea, and vomiting. Adverse events were graded according to standard toxicity grading scales (Supplemental Materials).
The protocol and informed consent forms were approved by the National Institute of Allergy and Infectious Diseases (NIAID), Division of Microbiology and Infectious Diseases (DMID), the US Food and Drug Administration, and the institutional review board of record for the study site.
Study vaccine
The vaccine antigen is an E. coli – produced recombinant protein which is formulated and lyophilized to yield Sm-p80 for injection. Sm-p80 stands for S. mansoni calpain protein with a mass of approximately 80 kDa and GLA-SE stands for Glucopyranosyl Lipid Adjuvant, a synthetic Toll-like receptor 4 agonist Monophosphoryl Lipid A – like molecule, which is formulated in a Squalene oil-in-water Emulsion. The antigen was manufactured by PAI Life Sciences Inc (Seattle, WA), the GLA-SE adjuvant was manufactured by AAHI (formerly IDRI, Seattle, WA, and drug product was filled and lyophilized by Lyophilization Technology Inc. (Ivyland, PA). Vaccine formulations were prepared by study site research pharmacists by reconstituting the antigen with water-for-injection and, when applicable, mixing with the liquid adjuvant. Vaccines were administered by IM injection in the deltoid at a volume of 0.5 mL.
Immunogenicity assay
We collected venous blood samples at each vaccination visit (prior to administration of vaccine), at seven days after each vaccination, and at 124 days after the third vaccination. For Groups A, B, D, and E (vaccinations given 28 days apart) we collected an additional sample at 28 days after the third vaccination. For Group C (vaccinations given on Days 1, 29, and 180), we collected additional samples at 28 days after the second and third vaccinations. Serum samples were tested by a qualified total IgG endpoint titer enzyme-linked immunosorbent assay (ELISA) against the Sm-p80 protein (supplied by PAI) at the Seattle Children’s Research Institute (Seattle, WA) using methods previously described 19.
Statistical analysis
The sample size of 45 participants (9 per each of the five study groups) was selected to obtain preliminary estimates of safety and immunogenicity to guide future research and product development and was not designed to test any specific null hypothesis.
For IgG antibodies, the immunogenicity outcome measures included the proportion of participants who met the definition of seroconversion, defined as a fourfold rise from baseline, at 28 days after the first, second, and third study vaccinations, and the geometric mean titers (GMTs) at the sample collection timepoints.
Data availability
All data generated or analysed during this study are available from the authors upon request, including individual de-identified participant data (including data dictionary) and statistical code.
References
Schistosomiasis (Bilharzia), <https://www.who.int/health-topics/schistosomiasis#tab=tab_1> (2024).
LoVerde, P. T. Schistosomiasis. Adv. Exp. Med Biol. 1454, 75–105 (2024).
Yamey, G. et al. Vaccine value profile for schistosomiasis. Vaccine. 126020. https://doi.org/10.1016/j.vaccine.2024.05.068 (2024)
Mo, A. X. & Colley, D. G. Workshop report: Schistosomiasis vaccine clinical development and product characteristics. Vaccine 34, 995–1001 (2016).
Zhou, Y. & Podesta, R. B. Effects of serotonin (5HT) and complement C3 on the synthesis of the surface membrane precursors of adult Schistosoma mansoni. J. Parasitol. 75, 333–343 (1989).
Ahmad, G. et al. Preclinical Prophylactic Efficacy Testing of Sm-p80-Based Vaccine in a Nonhuman Primate Model of Schistosoma mansoni Infection and Immunoglobulin G and E Responses to Sm-p80 in Human Serum Samples From an Area Where Schistosomiasis Is Endemic. J. Infect. Dis. 204, 1437–1449 (2011).
Ahmad, G. et al. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine 27, 2830–2837 (2009).
Ahmad, G. et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol. Res 105, 1767–1777 (2009).
Karmakar, S. et al. Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons. Vaccine 32, 1296–1303 (2014).
Karmakar, S. et al. Use of an Sm-p80-Based Therapeutic Vaccine to Kill Established Adult Schistosome Parasites in Chronically Infected Baboons. J. Infect. Dis. 209, 1929–1940 (2014).
Molehin, A. J. et al. Cross-species prophylactic efficacy of Sm-p80-based vaccine and intracellular localization of Sm-p80/Sm-p80 ortholog proteins during development in Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Parasitol. Res. 116, 3175–3188 (2017).
Zhang, W. et al. Longevity of Sm-p80-specific antibody responses following vaccination with Sm-p80 vaccine in mice and baboons and transplacental transfer of Sm-p80-specific antibodies in a baboon. Parasitol. Res. 113, 2239–2250 (2014).
Zhang, W. et al. Fifteen Years of Sm-p80-Based Vaccine Trials in Nonhuman Primates: Antibodies From Vaccinated Baboons Confer Protection in vivo and in vitro From Schistosoma mansoni and Identification of Putative Correlative Markers of Protection. Front Immunol. 11, 1246 (2020).
Zhang, W. et al. Sm-p80-based schistosomiasis vaccine: double-blind preclinical trial in baboons demonstrates comprehensive prophylactic and parasite transmission-blocking efficacy. Ann. N. Y. Acad. Sci. 1425, 38–51 (2018).
Keitel, W. A. et al. A phase 1 study of the safety, reactogenicity, and immunogenicity of a Schistosoma mansoni vaccine with or without glucopyranosyl lipid A aqueous formulation (GLA-AF) in healthy adults from a non-endemic area. Vaccine 37, 6500–6509 (2019).
Igetei, J. E., El-Faham, M., Liddell, S. & Doenhoff, M. J. Antigenic cross-reactivity between Schistosoma mansoni and peanut: a role for cross-reactive carbohydrate determinants (CCDs) and implications for the hygiene hypothesis. Immunology 150, 506–517 (2017).
Kifle, D. W. B. et al. A Functional Enzymatic Assay as Potential Readout for a Clinical Trial of a Schistosomiasis Vaccine. NPJ Vaccines 10, 48 (2025).
Diemert, D. J. et al. A randomized, controlled Phase 1b trial of the Sm-TSP-2 Vaccine for intestinal schistosomiasis in healthy Brazilian adults living in an endemic area. PLoS Negl. Trop. Dis. 17, e0011236 (2023).
Larsen, S. E. et al. Qualification of ELISA and neutralization methodologies to measure SARS-CoV-2 humoral immunity using human clinical samples. J. Immunol. Methods 499, 113160 (2021).
Acknowledgements
We thank the many contributors from NIAID who provided advice and assistance for this trial (Mohamed Elsafy, Lee Hall, Walter Jones, Brenda Larkin, Annie Mo, Aya Nakamura, Seemi Patel, Blossom Smith, and Michelle Wildman). We also thank the members of the Safety Monitoring Committee (Robert L Atmar,[chair], Kirsten E Lyke, and DeAnna J Friedman-Klabanoff) for their oversight, and the participants themselves for their altruism and dedication to this trial.
Author information
Authors and Affiliations
Contributions
L.A.J. had primary responsibility for development of the protocol, provided oversight of the conduct of this single-site trial at Kaiser Washington, and is the primary author for this manuscript. R.N.C., T.P., and S.E.L. provided scientific oversight and laboratory support of ELISA testing of blood samples. D.C., S.A.G., and J.D. were primarily responsible for technical issues regarding support for manufacture of the antigen and adjuvant and provided scientific expertise for the manuscript. G.A.D. provided scientific expertise for development of the protocol and the manuscript and G.A.D. and R.G. provided oversight of the conduct of the trial. C.M.P. provided oversight for the immunogenicity assays and scientific support. C.C., A.W., and J.S.L. provided analytic support for collection, recording and analysis of the safety and immunogenicity outcomes. A.A.S. and A.K. provided scientific support related to the study product and analyses of results. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
G.A.D. is currently an employee of AstraZeneca. D.C. is a shareholder in PAI Life Sciences Inc. which holds a license to the SchisoShield® product.All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jackson, L.A., Coler, R.N., Deye, G.A. et al. Safety and immunogenicity of the Sm-p80 GLA-SE schistosomiasis vaccine. npj Vaccines 10, 247 (2025). https://doi.org/10.1038/s41541-025-01261-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01261-3