Abstract
Syphilis, caused by Treponema pallidum, remains a major global health burden. Given the key role of T cell–mediated immunity in T. pallidum clearance, this study evaluates a Tp0136-derived T-cell epitope (Tp0136T1) delivered via two platforms: lipid nanoparticle–encapsulated nucleoside-modified mRNA (LNP-mRNA) and Pyrococcus furiosus thioredoxin (PfTrx). In BALB/c mice, both platforms elicited robust Th1-type responses, with increased IL-2 and IFN-γ secretion and activation of Th1 CD4⁺ T cell. Only LNP-mRNA-Tp0136T1 induced strong CD8⁺ cytotoxic responses, marked by elevated perforin⁺ and granzyme B⁺ expression. In New Zealand White rabbits challenged intradermally with T. pallidum, complete ulcer prevention was achieved with the PfTrx-Tp0136T1, while LNP-mRNA-Tp0136T1 significantly reduced ulceration. Both vaccines suppressed RPR titers and lowered treponemal load. These findings demonstrate that epitope-specific T cell–based vaccines elicit potent cellular immunity, control treponemes, prevent ulcers, and may reduce secondary sexually transmitted infections by preserving mucosal integrity.
Similar content being viewed by others
Introduction
Syphilis is a chronic, multistage sexually transmitted infection caused by Treponema pallidum (T. pallidum)1,2,3,4,5. Despite the availability of effective antibiotics, condom promotion, and the scale-up of rapid diagnostic testing, global syphilis incidence has continued to rise, affecting nearly 10 million individuals annually, including 1.4 million pregnant women3,6,7. This resurgence has led to a parallel increase in maternal and congenital syphilis, posing a serious threat to reproductive and neonatal health8. The ongoing epidemic underscores an urgent need for a safe and effective vaccine9. Recently, doxycycline post-exposure prophylaxis (doxy-PEP) has demonstrated real-world effectiveness in reducing incident syphilis cases among high-risk populations, underscoring the potential of preventive interventions to complement vaccine development10. Syphilis transmission occurs primarily through sexual contact, with infectivity peaking during the primary and secondary stages due to high treponemal loads2. Primary syphilis is marked by ulcerative lesions that harbor abundant T. pallidum, significantly compromising mucosal integrity and enhancing transmission—not only of syphilis but also of other sexually transmitted infections (STIs), including HIV11. Experimental rabbit models have shown that higher bacterial burdens at lesion sites correlate with increased tissue damage, elevated rapid plasma regain (RPR) titers, and systemic spread12,13. Thus, the ability to prevent ulcer formation and reduce treponemal burden is one of the key indicators of effective vaccine-induced protection14.
Despite sustained efforts, syphilis vaccine development remains in the preclinical stage, with most candidates demonstrating only partial protection in animal models15. Most syphilis vaccine studies have primarily focused on eliciting humoral immunity15. However, unlike intracellular pathogens, T. pallidum is an extracellular bacterium, and antibody-mediated responses alone appear insufficient for effective control16. While CD4⁺ T cells are widely recognized to play a central role in orchestrating Th1-type immunity, the contribution of CD8⁺ T cells in syphilis infection remains unclear, as their role in defense against an extracellular pathogen is not yet fully understood. Tp0136, a fibronectin-binding adhesin involved in syphilis early dissemination17, was the target of our previous work evaluating a T cell epitope–based vaccine. In that study, peptide vaccines encoding T cell epitopes conferred partial protection in rabbits, whereas B cell epitope formulations were ineffective18. Notably, prior studies of full-length Tp0136 have yielded mixed results, with some reporting protective effects19,20 and others describing exacerbation of lesion pathology19. Our recent dissection of Tp0136 into discrete immunological components provided a mechanistic explanation for these divergent findings: B cell epitopes were associated with aggravated pathology, whereas T cell epitopes mediated protection18,21. Together, these data highlight both the potential and the complexity of Tp0136 as a vaccine antigen, underscoring the critical importance of exploring antigen delivery platforms capable of optimizing immune activation18. These findings underscore the need to optimize antigen delivery systems that enhance MHC class I and II presentation and drive robust cellular immunity22.
To address this, we explored two complementary delivery platforms with distinct immunological profiles. The Pyrococcus furiosus thioredoxin (PfTrx) scaffold, a highly thermostable recombinant protein carrier, offers robust epitope display and has been reported to promote T cell activation even in the absence of adjuvants23. In contrast, lipid nanoparticle (LNP)-encapsulated nucleoside-modified mRNA represents a genetic vaccine platform that enables endogenous antigen synthesis and potent T cell stimulation through improved translation efficiency and cellular uptake24,25,26. While PfTrx scaffolds have been tested in only three published syphilis vaccine studies—targeting Tp0326, Tp0865, Tp0856, and Tp085827,28,29—to our knowledge, no mRNA-based vaccine has yet been reported in the context of syphilis research30. Given the success of mRNA platforms in viral and oncologic applications, their potential for eliciting protective T cell responses against T. pallidum warrants urgent investigation31.
Building on our previous findings that a T cell epitope derived from Tp0136 conferred partial protection in rabbits, we developed two next-generation vaccine candidates encoding the same epitope: (1) a recombinant PfTrx-Tp0136T1 protein and (2) a lipid nanoparticle–encapsulated, nucleoside-modified mRNA construct (Fig. S1). These platforms represent recombinant protein and genetic vaccine expression systems, respectively, and offer complementary strategies for enhancing intracellular antigen delivery and T cell activation. In this study, we systematically compare their immunogenicity and protective efficacy in two models: Balb/c mice, for detailed immunological profiling, and New Zealand White rabbits, for T. pallidum challenge-based protection assessment (Fig. 1). Our goal is to identify immune correlates of protection, elucidate the contribution of Th1-skewed cellular responses, and provide mechanistic insights to inform the design of T cell–focused syphilis vaccines.
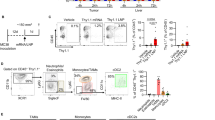
This schematic illustrates the comparative immunological effects of two Tp0136T1-targeted T cell epitope vaccines delivered via distinct expression platforms: an mRNA-based formulation encapsulated in lipid nanoparticles (LNP-mRNA-Tp0136T1) and a recombinant protein fused to Pyrococcus furiosus thioredoxin (rPfTrx-Tp0136T1). In murine studies, both candidates stimulated Th1-skewed immune responses, while the mRNA formulation additionally promoted robust CD8⁺ cytotoxic T cell activity. In rabbit challenge models, both vaccines lowered local treponemal loads and reduced RPR titer. The rPfTrx-Tp0136T1 vaccine offered complete protection against ulcer development, whereas the LNP-mRNA vaccine partially reduced ulcer incidence. Created with BioRender.com.
Results
Design, development, and validation of Tp0136T1 mRNA, protein, and peptide vaccine platforms
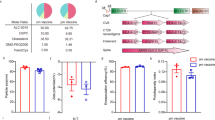
The LNP-mRNA-Tp0136T1 construct was engineered to express a codon-optimized immunogen encoding the T-cell epitope 1 (T1) epitope of the T. pallidum Tp0136 protein (L477–S486). The complete mRNA structure—comprising a 5′ cap, optimized untranslated regions (UTRs), and a 3′ poly(A) tail—was synthesized by Perfect mRNA Co. Ltd. (Hangzhou, China) and subsequently encapsulated into lipid nanoparticles (LNPs) using standardized manufacturing protocols. Capillary electrophoresis demonstrated that 89.6% of the transcripts formed a dominant peak at 213 nucleotides (Fig. S2), within the regulatory benchmark of 80–95% intact mRNA. LC-MS analysis verified a capping efficiency of 95.1% (Fig. S3A, B), and polyadenylation was confirmed via fragment length analysis showing a predominant tail length of 65–76 adenosines (Fig. S3C, D). Dynamic light scattering (DLS) revealed a monodisperse distribution with a mean hydrodynamic diameter ≤150 nm and polydispersity index (PDI) < 0.2 (Fig. 2A, Fig. S4A). Electrophoretic light scattering (ELS) indicated that the zeta potential was within the pharmaceutically acceptable range (−50 mV to +50 mV), supporting colloidal stability in biological systems (Fig. S4B). Encapsulation efficiency was determined via Triton X-100–mediated disruption assays and RiboGreen fluorescence, yielding 93.47% efficiency (Fig. 2B–D), exceeding the typical 90% threshold required for LNP-mRNA vaccines. Homogenized vaccine end products are illustrated in Fig. 2E. LNP-mRNA-Tp0136T1 exhibited excellent structural integrity, encapsulation efficiency, and nanoparticle properties suitable for prophylactic mRNA vaccine delivery. Indirect immunofluorescence was employed to evaluate the in vitro expression of Tp0136T1 following transfection with LNP-mRNA-Tp0136T1. Strong green fluorescence signals were observed in HEK-293T cells, confirming successful translation of the Nichols strain-specific Tp0136T1 antigen. The fluorescence intensity correlated with increasing concentrations of mRNA, indicating dose-dependent protein expression (Fig. 2F).
A Dynamic light scattering (DLS) analysis revealed a monodisperse nanoparticle distribution with a mean hydrodynamic diameter ≤150 nm and a polydispersity index (PDI) < 0.2. B–D Encapsulation efficiency of LNP-mRNA-Tp0136T1 was assessed by Triton X-100–mediated disruption and RiboGreen fluorescence, yielding 93.47%, above the 90% threshold for LNP-mRNA formulations. E Image of the homogenized LNP-mRNA-Tp0136T1 vaccine formulation and SDS-PAGE analysis of purified rPfTrx-Tp0136T1 protein showing >90% purity; Western blot confirmed the expected ~18 kDa molecular weight in both lanes. F Indirect immunofluorescence in HEK-293T cells demonstrated successful, dose-dependent expression of the Tp0136T1 antigen following mRNA transfection.
To generate a recombinant protein vaccine, the Tp0136T1 epitope was fused to the C-terminus of a Pyrococcus furiosus thioredoxin (PfTrx) scaffold with a 6×His tag. The construct was cloned into a pET28a (+) expression vector, expressed in E. coli BL21 (DE3) under IPTG induction, and purified via Ni-NTA chromatography. SDS-PAGE analysis confirmed >90% purity of the recombinant fusion protein, and Western blot analysis verified the expected molecular weight of approximately 18 kDa (Fig. 2E). This protein was used in subsequent animal immunizations and in vitro stimulation assays.
The T1 was selected based on comprehensive immunoinformatic predictions. Cytotoxic T lymphocyte (CTL) epitopes were identified using NetCTL 1.2 and IEDB Class I tools, while helper T lymphocyte (HTL) epitopes were predicted via IEDB Class II and IFNepitope servers. Topological orientation was assessed using the TMHMM server, confirming that the T1 epitope is extracellularly exposed. Antigenicity and allergenicity were evaluated using VaxiJen v2.0 and AllerTOP v2.0, respectively, indicating that T1 is a probable antigen and non-allergen. Chemically synthesized by Shanghai Sangon Biotechnology Co., Ltd. (China), the peptide was validated by high-performance liquid chromatography (HPLC) and mass spectrometry. The main chromatographic peak accounted for 99.709% of the total area and 99.651% of the total height (Fig. S5A, B). The observed molecular weight was 1303.30 Da, which closely matched the theoretical mass of 1304.492 Da (Fig. S5C). Tp0136T1 peptide was successfully conjugated to keyhole limpet hemocyanin (KLH) using sulfo-SMCC, yielding a stable immunogen formulation at 1 mg/mL.
Tp0136T1-based vaccines elicit distinct humoral and Th1-biased cellular immune responses in mice
To evaluate the immunogenicity of the Tp0136T1 epitope across different expression systems, BALB/c mice (n = 5–6 per group; mRNA group n = 6, others n = 5) were immunized with either LNP-mRNA-Tp0136T1 (genetic vaccine expression) or recombinant PfTrx-Tp0136T1 protein (protein vaccine expression). Control groups received LNP formulation alone or normal saline (Mock). Immunizations were administered at 21-day intervals, and serum samples were collected on days 42 and 56 post-initial immunization for analysis of humoral responses (Fig. 3A). Both LNP-mRNA-Tp0136T1 and rPfTrx-Tp0136T1 vaccines induced significantly higher endpoint antibody titers compared to the LNP and Mock control groups (Fig. 3B). Animals with endpoint titers ≥1:10,000 were humanely euthanized in accordance with experimental protocol. Both mRNA and recombinant protein vaccines targeting Tp0136T1 generated robust antigen-specific antibody responses in mice.
A Immunization and sampling schedule in BALB/c mice (n = 5–6 per group). Created with BioRender.com. B Endpoint serum IgG titers against Tp0136T1 peptide at week 8 showed significantly elevated antibody levels in both LNP-mRNA-Tp0136T1 and rPfTrx-Tp0136T1 groups compared to controls. C–F Cytokine profiling of splenocyte supernatants stimulated with rTp0136T1 protein. Both vaccine groups exhibited Th1-skewed responses, with significantly higher IL-2 (C) and IFN-γ (D) secretion compared to IL-4 (E) and IL-17A (F). IL-2 levels were modestly but significantly higher in the rPfTrx-Tp0136T1 group than in the mRNA group (p < 0.05). Low IL-4 levels were also detected in the LNP-alone group. G T cell proliferation assessed via EdU incorporation assay following in vitro antigen stimulation. Both vaccine groups showed enhanced T cell proliferation compared to controls, indicating effective T cell priming.
To evaluate Tp0136T1-specific T cell responses, splenocytes were harvested two weeks after the third immunization, stimulated in vitro with rTp0136T1 protein, and analyzed using indirect ELISA, EdU incorporation assay, and flow cytometry. Secreted cytokine levels were quantified via indirect ELISA. Both LNP-mRNA-Tp0136T1 and rPfTrx-Tp0136T1 vaccines elicited the production of interleukin-2 (IL-2) (Fig. 3C), interferon-γ (IFN-γ) (Fig. 3D), interleukin-4 (IL-4) (Fig. 3E), and interleukin-17A (IL-17A) (Fig. 3F). Among these, the levels of IL-2 and IFN-γ were markedly higher than those of IL-4 and IL-17A, indicating a Th1-skewed response. Notably, IL-2 secretion was slightly but significantly higher in the rPfTrx-Tp0136T1 group compared to the LNP-mRNA-Tp0136T1 group (p < 0.05, Fig. 3C). Interestingly, unlike other cytokines, a low level of IL-4 production was also observed in the LNP-alone control group (Fig. 3E), suggesting mild background activation. Both mRNA and protein-based Tp0136T1 vaccines predominantly activated Th1-type cytokines, with stronger IL-2 responses observed in the protein group.
Splenic T lymphocytes isolated from mice immunized with either LNP-mRNA-Tp0136T1 or rPfTrx-Tp0136T1 displayed robust proliferative responses upon antigen stimulation, showing statistically significant differences compared to the control group (Fig. 3G). These results indicate that both the mRNA-based and recombinant protein-based vaccine candidates effectively prime T cell-mediated immune responses.
Tp0136T1 vaccines induce distinct CD4⁺ and CD8⁺ T cell activation and functional polarization
Surface cytokine staining for CD4⁺ T cells expressing CD25, CD62L, and CD69 was performed to assess the activation status following immunization (Fig. 4A). Flow cytometric analysis revealed a differential pattern of activation marker expression after the third dose. Specifically, a higher proportion of CD4⁺ T cells expressed CD25 (Fig. 4D) compared to CD62L (Fig. 4E) and CD69 (Fig. 4F), in mice immunized with LNP-mRNA-Tp0136T1, rPfTrx-Tp0136T1, or LNP-only formulations.
A Representative flow cytometry gating strategy for surface activation markers on CD4⁺ T cells. D–F Quantification of CD25⁺, CD62L⁺, and CD69⁺ CD4⁺ T cells following immunization. B Gating strategy for intracellular cytokine staining to assess CD4⁺ T helper cell polarization. G–I Frequencies of IL-4⁺, IFN-γ⁺, and IL-17A⁺ CD4⁺ T cells following stimulation. C Gating strategy for intracellular staining of CD8⁺ T cells expressing granzyme B and perforin. J–K Quantification of granzyme B⁺ and perforin⁺ CD8⁺ T cells.
Intracellular cytokine staining of CD4⁺ T cells was performed to assess T helper cell polarization following immunization (Fig. 4B). A higher percentage of IL-4⁺ (Fig. 4G) and IFN-γ⁺ (Fig. 4H) CD4⁺ T cells was observed in mice immunized with LNP-mRNA-Tp0136T1, rPfTrx-Tp0136T1, or LNP-only formulations compared to the Mock group, suggesting the induction of both Th2 and Th1 responses. Interestingly, elevated IL-17A expression, indicative of a Th17 response, was only detected in the rPfTrx-Tp0136T1 group (Fig. 4I). These results demonstrate that Tp0136T1-based immunization activates multiple CD4⁺ T cell subsets, with distinct Th1, Th2, and Th17 polarization depending on the vaccine formulation.
To evaluate cytotoxic T lymphocyte (CTL) responses, intracellular staining was performed for perforin and granzyme B in CD8⁺ T cells (Fig. 4C). Notably, only the LNP-mRNA-Tp0136T1 group induced a significantly higher proportion of CD8⁺ T cells co-expressing granzyme B⁺ (Fig. 4J) and perforin⁺ (Fig. 4K) compared to the rPfTrx-Tp0136T1, LNP-only, and Mock groups. This indicates a strong CTL-inducing capacity of the mRNA-based platform. LNP-mRNA-Tp0136T1 selectively enhances cytotoxic CD8⁺ T cell responses, highlighting its potential for inducing protective cellular immunity.
To explore the relationships among key immune parameters, Spearman correlation analysis was conducted. In both the LNP-mRNA-Tp0136T1 (Fig. S6) and rPfTrx-Tp0136T1 (Fig. S7) groups, IL-2 levels showed a strong positive correlation with IFN-γ expression (p < 0.001) and antigen-specific T cell proliferative responses (p < 0.01 in LNP-mRNA-Tp0136T1, p < 0.001 in rPfTrx-Tp0136T1), indicating a coordinated activation of Th1-type immunity. IFN-γ itself was also positively correlated with proliferative responses (p < 0.01 in LNP-mRNA-Tp0136T1, p < 0.001 in rPfTrx-Tp0136T1), further supporting its central role in T cell-mediated activation. In the LNP-mRNA-Tp0136T1 group, perforin and granzyme B expression in CD8⁺ T cells were positively correlated (p < 0.001), reflecting coordinated cytotoxic effector function. These correlations highlight a tightly linked network of cellular immune responses induced by Tp0136T1-based vaccines, with IFN-γ and IL-2 serving as central nodes in orchestrating effective immunogenicity.
Tp0136T1-based vaccines confer partial protection against T. pallidum challenge in rabbit models
New Zealand White (NZW) rabbits were immunized with one of the following formulations: LNP-mRNA-Tp0136T1 (mRNA), recombinant PfTrx-Tp0136T1 protein (protein), or Tp0136T1 synthetic peptide (polypeptide), with the LNP-only group serving as a control. Immunizations were administered at 21-day intervals. To assess the humoral immune response, serum samples were collected at Week 6, corresponding to 21 days after the second dose (Fig. 5A). Animals exhibiting endpoint antibody titers of ≥1:10,000 were selected for intradermal challenge with 1 × 10⁵ T. pallidum Nichols strain organisms at eight dorsal sites (Fig. 5B). Lesion development and systemic clinical indicators were subsequently monitored over a 3-week observation period. This immunization-challenge model enables direct evaluation of vaccine-induced protection against T. pallidum infection through both serological and clinical parameters.
A Immunization and sampling schedule in New Zealand White (NZW) rabbits. Created with BioRender.com. B LNP-mRNA-Tp0136T1, rPfTrx-Tp0136T1 and peptide-Tp0136T1 induced significantly higher endpoint antibody titers compared to the LNP-only group (p < 0.05). C Treponemal DNA burden at lesion sites measured by qPCR. Skin biopsy samples were obtained from 2 randomly selected challenge lesions per rabbit (n = 3 rabbits per group). Vaccinated groups showed a significantly reduction in T. pallidum DNA burden compared with LNP controls (p < 0.05). D TPPA titers at 2 weeks post-challenge confirmed infection in all groups. E RPR titers revealed reduced serological response in the mRNA and protein vaccine groups compared to LNP-only and peptide groups (p < 0.05), indicating attenuated tissue damage. F, G Ulceration incidence across groups, with the rPfTrx-Tp0136T1 group showing complete protection (0%), and the mRNA group showing partial protection (16.7%). H Correlation between lesion diameter and TPPA titers or days post-challenge, demonstrating a relationship between humoral response and lesion progression. I Linear mixed-effects model showing ulcer count positively associated with RPR titers and negatively associated with rPfTrx-Tp0136T1 vaccination (p < 0.05). (J) Correlation heatmap showing strong associations among lesion treponemal load, RPR titer, and ulcer count (p < 0.05), underscoring their utility as composite markers of vaccine-mediated protection.
Our previous work has characterized Tp0136T1-specific T cell responses in the rabbit model18. In the current study, we aimed to assess the protective efficacy of different vaccine formulations by evaluating syphilis-related serological biomarkers. The T. pallidum particle agglutination (TPPA) assay was used to confirm successful infection, as it detects antibodies specific to T. pallidum. TPPA titers reached 1:320–1:640 in all immunized groups by Week 2 post-challenge, with no statistically significant differences observed among the four groups (Fig. 5D). In contrast, the rapid plasma reagin (RPR) test, which reflects tissue damage associated with active infection, showed significantly elevated titers in the LNP-only and peptide groups (1:16–1:32), compared to the LNP-mRNA-Tp0136T1 and rPfTrx-Tp0136T1 groups (1:4–1:8; p < 0.05; Fig. 5E). Higher RPR titers typically indicate more severe tissue injury and inflammatory responses. These results suggest that both the mRNA and protein vaccines attenuated disease severity, as evidenced by reduced RPR titers despite comparable infection rates across groups.
T. pallidum burdens at the skin inoculation sites were significantly reduced in the mRNA, peptide, and recombinant protein groups relative to the LNP control group (p < 0.05; Fig. 5C), supporting effective suppression of local bacterial replication. Although no statistically significant differences were observed in the incidence of wheals and papules or in lesion diameters among the groups, ulceration rates varied markedly between vaccine formulations (Fig. 5F, G). The mRNA (16.7%) and recombinant protein (0%) groups exhibited substantially lower ulceration rates compared to the LNP-only (75%) and peptide (33.3%) groups, indicating superior control of lesion progression in the former. These findings demonstrate that the mRNA and protein vaccines confer partial protection by limiting ulcerative lesion development and reducing local T. pallidum loads.
Lesion diameter was significantly associated with TPPA titers (p < 0.001) and days post-challenge (p < 0.05; Fig. 5H), indicating a link between humoral responses and lesion progression. A linear mixed-effects model analysis of ulcerative lesion numbers revealed a positive association with RPR titers and a significant negative association with recombinant rPfTrx-Tp0136T1 protein immunization (p < 0.05; Fig. 5I), suggesting that higher disease activity and lack of protective vaccination contribute to lesion severity.
To better understand factors driving ulcer development, rabbits were stratified into “ulcerative” and “non-ulcerative” groups. In the ulcerative subgroup, Spearman correlation analysis showed that TPPA titers had a moderate, non-significant correlation with ulcer number (rs = 0.399, p = 0.224), while RPR titers exhibited a strong and significant positive correlation (rs = 0.831, p < 0.05; Fig. S8A). Further stratification based on RPR levels (high: ≥1:8; low: <1:8) provided additional insights. In the high-RPR group, TPPA titers were weakly and non-significantly correlated with ulcer count (rs = 0.331, p = 0.468), whereas RPR remained strongly correlated (rs = 0.842, p < 0.05). In the low-RPR group, both TPPA (rs = 0.596, p < 0.05) and RPR (rs = 0.486, p < 0.05) were significantly associated with ulcer number (Fig. S8B). These analyses underscore RPR titers as a robust indicator of ulcer severity, while the association of TPPA with lesion outcomes appears to depend on overall disease activity.
A linear mixed-effects model was utilized to evaluate the impact of TPPA titers, RPR titers, days post-challenge, and vaccine formulation on the number of ulcerative lesions (Table S1). The analysis identified RPR titer and vaccine type as significant predictors of ulcer formation. Specifically, each unit increase in RPR titer was associated with an average increase of 0.735 ulcers (p < 0.05), highlighting RPR as a strong indicator of disease severity. In contrast, immunization with rPfTrx-Tp0136T1 reduced the number of ulcers by 1.675 lesions compared to the LNP-only control (p < 0.05). No statistically significant reduction in ulcer count was observed in the LNP-mRNA-Tp0136T1 and the Peptide-Tp0136T1group relative to the LNP-only group. TPPA titers and days post-challenge showed no significant associations with ulcer number. Correlation heatmap analysis further confirmed that lesion T. pallidum burden, RPR titers, and ulcer count were significantly correlated (p < 0.05; Fig. 5J), supporting the relevance of these parameters as interconnected markers of disease progression. These findings reinforce RPR titers and bacterial load as key predictors of lesion severity, while recombinant protein and mRNA vaccines demonstrate significant protective effects by reducing ulcer formation.
Discussion
In this study, we evaluated two antigen delivery platforms—LNP-encapsulated mRNA and recombinant PfTrx-fused protein—encoding a Tp0136-derived T cell epitope, and compared their immunogenicity and protective efficacy in murine and rabbit models of T. pallidum challenge respectively. Both vaccines induced robust Th1-skewed immune responses in mice, characterized by elevated IL-2 and IFN-γ production and enhanced T cell activation. In rabbits, immunization led to reduced T. pallidum burden at lesion sites, significantly fewer ulcerations, and slower increases in RPR titers, indicating attenuated tissue damage and systemic disease activity. Notably, the rPfTrx-Tp0136T1 vaccine provided superior clinical protection, completely preventing ulcer formation. In contrast, the LNP-mRNA-Tp0136T1 vaccine may elicited stronger cytotoxic CD8⁺ T cell responses and contribute to inflammatory pathology, with fewer ulcers than the LNP-only or peptide-Tp0136T1 groups but more than the rPfTrx group. These findings suggest that while cytotoxic responses aid bacterial clearance, excessive CD8⁺ activation fails to mitigate local tissue injury. Overall, our results highlight the protective role of T cell–mediated immunity in reducing treponemal burden, suppressing RPR titer elevation, and preventing ulcer development—while also emphasizing the importance of calibrating immune responses to balance efficacy with immunopathology in syphilis vaccine design.
The development of a syphilis vaccine has long been hindered by the unique biology of T. pallidum, which expresses a limited number of surface-exposed outer membrane proteins, many of which are structurally complex and difficult to purify32. Recent advances in reverse and structural vaccinology have facilitated the identification of promising outer membrane targets such as the Tpr family, FadL, and BamA, whose β-barrel structures mediate key functions including nutrient uptake and membrane integrity33,34,35,36. Antibodies targeting the extracellular loops of these proteins have demonstrated opsonophagocytic activity28, and with the advent of in vitro culture systems for T. pallidum, their functional roles can now be validated under controlled conditions29.Nonetheless, antibody-based protection appears to be insufficient, as the resolution of syphilitic lesions largely depends on T cell–mediated immunity, particularly delayed-type hypersensitivity (DTH) responses29. Our previous work highlighted this dichotomy: immunization with B cell epitopes from Tp0136 failed to confer protection and even exacerbated lesion severity, while T cell epitope–based immunization elicited strong Th1 responses and significantly reduced lesion progression in rabbits18. Notably, the PfTrx scaffold can efficiently present antigenic epitopes. Following fusion expression, antibodies with a higher titer may be generated as a result of cross - reactions or fusion epitopes. This, to a certain degree, potentiates the B cell response to the T1 epitope.
Building on these insights, the present study employed two distinct antigen delivery platforms to optimize epitope presentation: a recombinant PfTrx protein scaffold and a genetic LNP-encapsulated mRNA construct. While prior studies have incorporated PfTrx to present antigens such as Tp0326 and Tp085628, our work is the first to utilize this scaffold for a defined T cell epitope of Tp0136—a fibronectin-binding adhesin with established roles in host-pathogen interaction18. Moreover, this study represents the first application of LNP-mRNA technology in syphilis vaccine research, an approach previously validated in Borrelia burgdorferi models37,38. This dual-platform strategy not only enabled a direct comparison of recombinant protein versus genetic vaccine antigen expression systems but also provided mechanistic insights into how delivery modality influences the balance between protective immunity and immunopathology.
In comparing our findings with prior vaccine studies, it is noteworthy that multi-epitope formulations—such as the TprC/TprK/Tp0751 tri-antigen cocktail—conferred robust protection in animal models, effectively preventing the development of infectious chancres and markedly limiting systemic dissemination39. By contrast, in the present study, a single Tp0136 T-cell epitope delivered via the PfTrx scaffold achieved complete (100%) local protection, whereas the same epitope encoded in an LNP-mRNA construct conferred a more modest effect (83.3%). These discrepancies underscore both the promise and limitations of minimalist vaccine designs: while single epitopes may yield potent responses in select contexts, the low and variable antigenicity of T. pallidum suggests that broader antigen coverage will likely be required for robust and durable protection. Nevertheless, streamlined single-epitope strategies offer advantages in safety and translational feasibility, and could be rationally integrated into multi-antigen cocktails. Such hybrid approaches may synergize the breadth of multi-antigen protection with the precision and safety of focused epitope delivery, thereby informing next-generation vaccine strategies for syphilis.
The observed differences in protective efficacy between the two platforms likely stem from distinct immune activation profiles. While both vaccines induced strong Th1-skewed responses, only the LNP-mRNA-Tp0136T1 vaccine triggered substantial CD8⁺ T cell cytotoxicity, as evidenced by increased perforin⁺ and granzyme B⁺ populations. Although such cytotoxic responses may aid bacterial clearance, excessive activation fails to reduce and may even exacerbate local tissue injury13. In contrast, the rPfTrx-Tp0136T1 vaccine elicited a more balanced immune profile—dominated by Th1 CD4 + T cells responses and elevated IL-2/IFN-γ levels—without excessive cytotoxicity, resulting in effective control of T. pallidum burden with minimal ulceration. This finding echo earlier studies suggesting that DTH-mediated macrophage activation, rather than direct cytotoxicity, plays a central role in syphilis lesion resolution13. Therefore, the design of syphilis vaccines must carefully calibrate cellular immune effectors to optimize bacterial clearance while limiting immunopathological damage.
Beyond its direct implications for syphilis control, the ability of a vaccine to prevent ulcer formation carries broader significance for STI prevention19. Syphilitic ulcers create mucosal breaches that facilitate the transmission of other pathogens such as HIV40. By contrast, type IV (DTH-driven) urticarial lesions (wheals or papules) are more localized and less disruptive to epithelial integrity13. In our study, the reduction of ulcers in vaccinated animals suggests that T cell–focused vaccine strategies may preserve mucosal barriers and thereby reduce the risk of STI coinfection. This highlights an important secondary benefit of vaccine-mediated modulation of the host inflammatory response.
This study provides new insights into the potential of epitope-specific T cell–based vaccines against syphilis, while also highlighting areas that require further consideration. The rabbit challenge dose (8 × 10⁵ organisms) was intentionally set much higher than the estimated human ID₅₀ (~57 organisms via intradermal exposure)41, ensuring reproducible lesion development but limiting the extent to which the model reflects natural transmission. The number of animals was constrained by ethical requirements and the 3Rs principle42, and an unexpected death in the rPfTrx-Tp0136T1 group further reduced statistical power. Extending the observation period to 21 days allowed a more comprehensive evaluation of lesion progression; however, longer-term monitoring and assessment of systemic dissemination were not performed.
The design of control groups may also affect data interpretation. The LNP-only group did not include irrelevant mRNA, making it difficult to rule out potential contributions from non-specific RNA–induced innate responses. Similarly, the lack of PfTrx-only, AddaVax-only, or irrelevant epitope controls introduces uncertainty regarding scaffold-, adjuvant-, or antigen-specific effects. In addition, evaluating immunogenicity in mice while assessing protection in rabbits introduces interspecies variability43. For instance, CD8⁺ activity in rabbits was inferred from earlier Tp0136 studies rather than directly measured, and the assumption that DTH-driven macrophage activation accounted for bacterial clearance remains indirect without lesion histology or cytokine profiling.
The murine model itself presents challenges. BALB/c mice have a Th2-skewed background, which could limit generalizability despite robust Th1 cytokine induction. Confirmation in C57BL/6 or other strains will strengthen interpretation. In addition, no positive control antigen (e.g., OVA) was included, making it difficult to benchmark the magnitude of T-cell responses. The durability of immunity was also not addressed, since challenges were performed soon after boosting and memory responses were not assessed.
Nevertheless, both LNP-mRNA-Tp0136T1 and rPfTrx-Tp0136T1 vaccines targeting a Tp0136-derived T-cell epitope induced strong Th1-type responses and provided partial protection in rabbits. Interestingly, the mRNA platform generated more robust CD8⁺ cytotoxicity, whereas the PfTrx scaffold achieved superior clinical outcomes, highlighting the importance of balancing immune potency with safety. By preventing ulcer formation, these vaccines may not only reduce T. pallidum transmission but also lower the risk of secondary sexually transmitted infections, offering additional public health value. Future studies incorporating longer-term follow-up, additional control groups, lower-dose challenges, and broader animal models will be critical to refine and extend these findings.
Methods
Materials
SM102, DSPC, DMG-PEG and cholesterol were purchased from Perfect mRNA Co. Ltd., Hangzhou, China. Isopropyl-β-D-thiogalactopyranoside (IPTG), His-tag protein purification kit and Edu detection kit were purchased from Beyotime Biotechnology (China). Luria–Bertani medium and kanamycin were supplied by Shanghai Sangon Biotechnology Co., Ltd. Freund’s adjuvant acquired from Thermo Fisher Scientific (USA). The antibodies used for flow cytometry were listed in Table S2. ELISA kits for Mouse IL-2, Mouse IL-4, Mouse IL-17A, Mouse IFN-γ, and Mouse IL-13 were procured from RayBiotech Co., Ltd. (China). HRP-conjugated goat anti-mouse secondary antibody, HRP-conjugated goat anti-rabbit secondary antibody, FITC-conjugated goat anti-mouse fluorescent secondary antibody was sourced from Shanghai Sangon Biotechnology Co., Ltd. (China).
Peptide preparation
The T-cell 1 (T1) epitope of T. pallidum Tp0136 (LWKFDTTTCS, corresponding to amino acids 482–491) was synthesized by Sangon Biotech (Shanghai, China), as previously described18. The Tp0136 sequence was derived from T. pallidum strain Nichols (GenBank accession AE000520.1). Peptide was purified by high-performance liquid chromatography (HPLC) and verified by mass spectrometry. To enhance immunogenicity, the Tp0136T1 peptide was chemically conjugated to keyhole limpet hemocyanin (KLH) using the heterobifunctional crosslinker sulfo-SMCC. The lyophilized peptide was stored at −20 °C and reconstituted in sterile phosphate-buffered saline (PBS) prior to use. Sequence alignment showed that the T1 peptide is conserved in the clinically predominant Nichols and SS14 strains, as well as in Chicago, Bal73-1, Seattle81-4, and MexicoA. In contrast, three T. pallidum ssp. pertenue strains (Samoa D, CDC2, and Gauthier) and Fribourg-Blanc harbor a single amino acid substitution (LWKFDTTACS), whereas T. pallidum ssp. endemicum strain Bosnia A lacks the T1 peptide region.
Recombinant protein production
The recombinant PfTrx-Tp0136T1 protein was engineered by fusing the T1 epitope (LWKFDTTTCS) to the C-terminus of a Pyrococcus furiosus thioredoxin (PfTrx) scaffold and tagging it with a 6×His sequence. The fusion construct was cloned into the pET28a (+) expression vector and transformed into Escherichia coli. BL21 (DE3). Expression was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), and the recombinant protein was purified using Ni-NTA affinity chromatography. The final protein product was concentrated to 1 mg/mL in PBS with a purity >90%, as confirmed by SDS-PAGE and WB.
LNP-mRNA vaccine formulation and characterization
The codon-optimized mRNA encoding the Tp0136 T1 epitope (LWKFDTTTCS), flanked by a 5′ cap, 5′/3′ untranslated regions (UTRs), and a 3′ poly(A) tail, was synthesized by Perfect mRNA Co. Ltd. (Hangzhou, China). Designed as a minimal nucleoside-modified construct optimized for cytoplasmic translation, it included a 5′ UTR (TCGGCCTCTTCTGGTCCCCACAGACTCAGAGAGAACCC), a Kozak sequence (GCCACC) upstream of the start codon, and a coding region encoding the Tp0136T1 minimal CD8⁺ T-cell epitope (L482–S491). No signal peptide was incorporated to favor intracellular expression. The 3′ UTR (GCTCGCTTTCTTGCTGTCCAATTTCTATTAAAGGTTCCTTTGTTCCCTAAGTCCAACTACTAAACTGGGGGATATTATGAAGGGCCTTGAGCATCTGGATTCTGCCTAATAAAAAACATTTATTTTCATTGCCACCTGGAGCTAGC) was followed by a synthetic poly(A) stretch to enhance transcript stability. An N1-methyl-pseudouridine triphosphate (N1-Me-pUTP) cap was used to improve stability and translational efficiency.
The integrity of the mRNA was verified by capillary gel electrophoresis (CGE), while capping efficiency and poly(A) tail length were determined by liquid chromatography–mass spectrometry (LC-MS) following nuclease digestion. The mRNA was encapsulated in lipid nanoparticles (LNPs), and the resulting complexes were characterized by dynamic light scattering (DLS) and electrophoretic light scattering (ELS) to assess particle size, polydispersity index (PDI), and zeta potential. Encapsulation efficiency was measured using a RiboGreen fluorescence assay.
In vitro expression of Tp0136T1 from the LNP-mRNA vaccine
HEK-293T cells (ATCC® CRL-3216™) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. To assess the expression of Tp0136T1 protein, cells were seeded in 6-well plates at a density of 1 × 10⁵ cells per well and transfected with 0–3 µg of LNP-mRNA per well. After 48 h, cells were fixed and stained using an indirect immunofluorescence protocol. Sera from mice immunized with Tp0136-derived peptide conjugated to KLH served as the primary antibody, followed by FITC-conjugated Goat-anti-mouse Fc as the secondary antibody. The cytoskeleton was counterstained with TRITC-conjugated phalloidin. Fluorescence signals were visualized using a Nikon A1+ confocal laser scanning microscope.
Animals and ethical approval
Female BALB/c mice (6–8 weeks old) and adult male New Zealand White (NZW) rabbits (2.5–3.5 kg) were purchased from Guangdong Medical Laboratory Animal Breeding Center (Guangzhou, China). Rabbits were housed in the Animal Laboratory Center of South China Agricultural University under specific pathogen-free (SPF) conditions at a constant temperature of 18–20 °C and provided with sterilized water and antibiotic-free feed. Prior to immunization, all animals tested seronegative for T. pallidum by both treponemal (TPPA, FUJIREBIO Inc., Japan) and non-treponemal (RPR, Shanghai Kehua Bio-Engineering Co., Ltd., China) serologic tests.
All animal experiments were reviewed and approved by the Institutional Ethics Committee of Dermatology Hospital, Southern Medical University (approval No. GDDHLS-20181202).
Mouse immunization study design
Five to six female BALB/c mice were allocated to each experimental group and immunized on Days 0, 21, and 42. Mice in the LNP-mRNA-Tp0136T1 and LNP-only groups received 5 µg of mRNA or LNP control in a total volume of 50 µL via intramuscular (IM) injection. Mice in the recombinant PfTrx-Tp0136T1 protein groups were immunized intraperitoneally (IP) with 200 µL of complete Freund’s adjuvant (CFA) emulsion containing 50 ng of antigen for the primary dose, followed by booster immunizations with incomplete Freund’s adjuvant (IFA) emulsion containing 25 ng of antigen. Mice in the mock group received 50 µL of sterile saline (IM). Whole blood samples were collected on Days 21, 42, and 56 for serologic analysis of anti-Tp0136T1 antibodies. On Day 56 (2 weeks after the final immunization), mice were euthanized and splenocytes were isolated for assessment of T cell responses.
Rabbit immunization study design
Three adult male NZW rabbits were included per group and immunized on Days 0 and 21. The LNP-mRNA-Tp0136T1 and LNP-only groups received 100 µg of mRNA or control LNPs formulation without any RNA payload in a total volume of 1 mL via intramuscular injection. The peptide and recombinant PfTrx-Tp0136T1 protein groups received 800 µL of complete Freund’s adjuvant emulsion containing 400 ng of antigen subcutaneously (SC) for the primary immunization, followed by booster immunization with 800 µL of IFA containing 200 ng of antigen. Whole blood was collected on Days 21 and 42 to assess anti-Tp0136T1 antibody responses via serologic testing.
Animal blood collection and splenocyte isolation
For mice, interim blood samples were obtained via tail vein bleeding. Terminal bleeds were performed via cardiac puncture under anesthesia. For rabbits, blood was collected via the ear vein. Blood samples were allowed to clot at room temperature (RT) for 30 min, then centrifuged at 3000 rpm for 10 min to isolate serum, which was stored at −80 °C until analysis. Spleens were harvested on Day 56 from 5 mice per group and processed individually. Each spleen was mechanically dissociated using a 70 µm cell strainer (Fisher Scientific) into 5 mL of complete RPMI medium (RPMI 1640 + 10% FBS + 1% penicillin-streptomycin). Red blood cells (RBCs) were lysed using ACK buffer, and cell suspensions were filtered and washed. Trypan blue exclusion staining was used to determine cell viability and yield using a hemocytometer.
Analysis of humoral responses to Tp0136T1 vaccination
Enzyme-linked immunosorbent assays (ELISAs) were performed to assess the antibody responses against Tp0136T1. ELISA plates were coated with 1 µg/mL of recombinant Tp0136T1 protein - the protein used to test antibody specificity against the 10–amino acid epitope was from mice immunized with the Tp0136T1 peptide conjugated to KLH - in carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4 °C. After two washes with TBST (0.05% Tween-20 in TBS), wells were blocked with 5% skim milk in TBST for 90 min at 37 °C. Plates were incubated with 1:10,000-diluted mouse or rabbit serum for 1 h at 37 °C, followed by incubation with horseradish peroxidase (HRP)-conjugated Goat-anti-mouse IgG (1:8,000 dilution; Sangon Biotech, Shanghai, China) for 45 min at 37 °C. Color development was performed using TMB substrate solution (Bioss, Cat. No. C04-03002), and the reaction was terminated with TMB stop solution. Absorbance at 450 nm was measured within 20 min using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific).
Cellular proliferation assay (EdU incorporation)
Spleens were processed into single-cell suspensions and adjusted to 1 × 10⁶ cells per well in 24-well plates. Negative controls consisted of mock-stimulated cells (no peptide), while no dedicated positive control was included. Cells were stimulated with 10 μg/mL Tp0136T1 peptide for 24 h at 37 °C. Following stimulation, 300 µL (10 μM) of EdU-containing medium was added to each well and incubated for another 2 h. Cells were then fixed in 4% paraformaldehyde for 30 min, treated with 2 mg/mL glycine for 5 min, and permeabilized with 0.5% Triton X-100 for 10 min. EdU incorporation was detected using a commercially available EdU assay kit (Sangon Biotech, Shanghai, China) following the manufacturer’s protocol. Nuclei were counterstained with Hoechst 33342 for 20–30 min. EdU-positive cells were visualized and imaged by fluorescence microscopy.
Cytokine secretion assays
To evaluate antigen-specific cytokine release, splenocytes were plated at 1 × 10⁶ cells per well in 24-well plates and stimulated with 10 μg/mL Tp0136T1 peptide for 48 h at 37 °C in a humidified incubator with 5% CO₂. Mock stimulation with PBS served as the negative control, and each condition was tested in triplicate wells. After incubation, cell culture supernatants were collected and analyzed for IL-2, IL-4, IL-17A (pro-inflammatory isoform), and IFN-γ using commercially available ELISA kits (Absin, Shanghai, China) according to the manufacturer’s instructions. Cytokine concentrations were calculated using standard curves and expressed as pg/mL.
Flow cytometric analysis of surface and intracellular cytokines
To assess vaccine-induced T cell phenotypes, intracellular cytokine staining (ICS) was performed. Freshly isolated splenocytes (5 × 10⁶ cells/well) were cultured in complete RPMI 1640 medium with either Tp0136T1 peptide (0.1 μg/μL, stimulated group) or PBS (unstimulated control) for 4 h at 37 °C. Following stimulation, surface staining (CD3, CD4, CD8, CD25, CD62L, CD69) and intracellular marker staining were performed on the cells respectively. Intracellular marker staining requires the addition of PMA and Ionomycin for stimulation, followed by Brefeldin blocking and further staining. After the staining of surface markers was completed, membrane permeation treatment was carried out with BD IC fixation buffer for 30 min, then washed with BD Perm/Wash buffer, and stained for intracellular cytokines (IL-4, IFN-γ, IL-17A, perforin, granzyme B) using appropriate fluorochrome-conjugated antibodies. All antibodies were used at a working concentration of 50 μg/mL. Specifically, CD4⁺ T cells were assessed for IL-4, IFN-γ, and IL-17 to evaluate Th2, Th1, and Th17 polarization, whereas CD8⁺ T cells were primarily assessed for perforin and granzyme B to capture cytotoxic effector functions. IFN-γ was excluded from the CD8⁺ ICS panel because it was evaluated in the supernatant secretion assays and in the CD4⁺ ICS analysis, thereby allowing the CD8⁺ panel to focus on direct cytolytic activity. Dead cells were excluded using fixable viability dye eFluor™ 506 (Thermo Fisher Scientific), and nuclei were stained with DAPI. For each sample, 50,000 events were acquired on a BD FACSCelesta flow cytometer and analyzed using FlowJo™ software (BD Biosciences).
Propagation of T. pallidum and Rabbit Challenge Model
The T. pallidum Nichols strain was maintained in the Dermatology Hospital of Southern Medical University and propagated intratesticularly in NZW rabbits as previously described12. For experimental infection, rabbits were inoculated intradermally with 1 × 10⁵ T. pallidum organisms at eight dorsal skin sites. Rabbits were monitored daily for lesion development. On Day 21 post-infection, animals were euthanized, and lesion progression was recorded. Skin tissues were collected from 2 randomly selected lesion sites per rabbit (out of 8 lesions per animal) for DNA extraction and quantification of T. pallidum burden.
Quantification of T. pallidum DNA Load by TaqMan Probe-Based qPCR
Quantification of T. pallidum DNA was performed using a TaqMan probe-based quantitative PCR (qPCR) targeting the polA gene. The 25 μL reaction mixture contained 12.5 μL of 2× TaqMan Gene Expression Master Mix (Thermo Fisher Scientific), 2.5 μL of 2.5 μM forward/reverse primer mix, 2.5 μL of 2 μM FAM-labeled probe, 1 μL of 50 mM MgCl₂, 4 μL of nuclease-free water, and 2.5 μL of DNA template. Reactions were run on a CFX96 Real-Time PCR System (Bio-Rad) under the following conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A standard curve was generated using plasmids containing the polA gene to determine absolute copy numbers.
Treponema pallidum Particle Agglutination (TPPA) Assay
Serum samples were tested for antibodies against Treponema pallidum using a particle agglutination assay (TPPA; FUJIREBIO Inc., Japan), following the manufacturer’s instructions. Briefly, serum was serially diluted two-fold with the provided diluent, starting from 1:40 up to 1:20,480. Each dilution (25 μL) was added into V-bottom 96-well microtiter plates containing 25 μL of sensitized gelatin particles. Negative control particles were included in parallel. The plates were gently shaken and incubated at room temperature (15–30 °C) for 2 h. Agglutination patterns were visually interpreted: a diffuse lattice formation was considered positive, while a compact button at the well bottom was considered negative. The endpoint titer was defined as the highest dilution that yielded a positive reaction.
Rapid plasma reagin (RPR) test
Nontreponemal antibody detection was performed using a rapid plasma reagin (RPR) test kit (Shanghai Kehua Bio-Engineering Co., Ltd., China). Following the manufacturer’s protocol, 50 μL of undiluted serum was placed on a disposable card, and 20 μL of carbon antigen suspension was added. The card was rotated for 8 min at 100 rpm at room temperature (23–27 °C). The results were interpreted macroscopically: visible black clumping indicated a positive reaction, while a smooth gray suspension indicated a negative result. Semi-quantitative titers were determined by serial two-fold dilutions of serum (1:1 to 1:32 or higher) and repeating the procedure. The final titer was defined as the highest dilution showing a positive reaction.
Statistical analysis
Statistical analysis was performed using SPSS version 25.0 for Windows (IBMCorp., Armonk, N.Y., USA), GraphPad Prism version 8.3.0 for Windows (GraphPad Software, Inc., San Diego, California USA), and R version 4.2.3 for Windows (R Foundation for Statistical Computing, Vienna, Austria). Data are presented as mean ± standard deviation (SD). For comparisons among three or more groups, the one-way analysis of variance (ANOVA) was applied, and the Tukey’s multiple comparison test was used for pairwise comparisons. For comparisons of the proportion of ulcers at inoculation sites between groups, the Chi-square test was used with the Bonferroni method for pairwise comparison. Spearman correlations were used to analyze associations between lesion severity and serological markers. A linear mixed-effects model was used to analyze changes in lesion severity in immunized NZW rabbits at 7-, 14-, and 21-days post-challenge. The fixed effects included the days post-challenge, group (LNP-mRNA-Tp0136T1 vs LNP, Peptide-Tp0136T1 vs LNP, rPfTrx-Tp136T1 vs LNP), TPPA, and RPR, and a random effect was used for each NZW rabbit. A p-value < 0.05 was considered statistically significant.
Data availability
The raw data generated from this study can be made available upon reasonable request.
References
Papp, J. R., Park, I. U., Fakile, Y., Pereira, L., Pillay, A. & Bolan, G. A. CDC laboratory recommendations for syphilis testing, United States,. Mmwr. Recommen. Rep. 73, 1–32 (2024).
Ramchandani, M. S., Cannon, C. A. & Marra, C. M. Syphilis. Infect. Dis. Clin. North Am. 37, 195–222 (2023).
Ghanem, K. G., Ram, S. & Rice, P. A. The modern epidemic of syphilis. N. Engl. J. Med 382, 845–54 (2020).
Zhou, S. & Chanderraj, R. What is syphilis?. JAMA 329, 1710 (2023).
The Past 70 Years in Control of Syphilis in China: Elimination and Responses to Resurgence. Chen, Xiang-Sheng; Jiang, Ting-Ting; Yin, Yue-Ping; More International Journal of Dermatology and Venereology. 3:193-197, December 2020.
Peeling, R. W., Mabey, D., Chen, X. S. & Garcia, P. J. Syphilis. Lancet 402, 336–46 (2023).
Newman, L., Kamb, M., Hawkes, S., Gomez, G., Say, L., Seuc, A. et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med 10, e1001396 (2013).
Buonsenso, D., Raffaelli, F., Camporesi, A., Fiori, B., Ricci, R. & Romano, L. Neonatal outcomes of mothers with syphilis during pregnancy: a retrospective single center experience. Children (Basel) 12, 307 (2025).
Kojima, N., Konda, K. A. & Klausner, J. D. Notes on syphilis vaccine development. Front Immunol. 13, 952284 (2022).
Sankaran, M., Glidden, D. V., Kohn, R. P., Nguyen, T. Q., Bacon, O., Buchbinder, S. P. et al. Doxycycline postexposure prophylaxis and sexually transmitted infection trends. JAMA Intern Med. 185, 266–72 (2025).
Lynn, W. A. & Lightman, S. Syphilis and HIV: a dangerous combination. Lancet Infect. Dis. 4, 456–66 (2004).
Huang, J., Jiang, Y., Lin, W., Chen, R., Zhou, J., Guo, S. et al. Virulence and adhesion of the treponema pallidum nichols strain simultaneously decrease in a Continuous-Infection new zealand white rabbit model. ACS Infect. Dis. 9, 1221–31 (2023).
Carlson, J. A., Dabiri, G., Cribier, B. & Sell, S. The immunopathobiology of syphilis: the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol. 33, 433–60 (2011).
Waugh, S. & Cameron, C. E. Syphilis vaccine development: Aligning vaccine design with manufacturing requirements. Hum. Vaccin Immunother. 20, 2399915 (2024).
Avila-Nieto, C., Pedreno-Lopez, N., Mitja, O., Clotet, B., Blanco, J. & Carrillo, J. Syphilis vaccine: challenges, controversies and opportunities. Front Immunol. 14, 1126170 (2023).
Lafond, R. E. & Lukehart, S. A. Biological basis for syphilis. Clin. Microbiol. Rev. 19, 29–49 (2006).
Li, W., Luo, X., Zheng, X. Q., Li, Q. L., Li, Z., Meng, Q. Q. et al. Treponema pallidum protein Tp0136 promotes angiogenesis to facilitate the dissemination of Treponema pallidum. Emerg. Microbes Infect. 13, 2382236 (2024).
Li, Q. L., Li, W., Zheng, X. Q., Ye, W. M., Xu, Q. Y., Ke, W. J. et al. Screening the B- and T-cell epitope map of TP0136 and exploring their effect in a Treponema pallidum rabbit model. Biomed. Pharmacother. 167, 115628 (2023).
Xu, M., Xie, Y., Zheng, K., Luo, H., Tan, M., Zhao, F. et al. Two Potential syphilis vaccine candidates inhibit dissemination of treponema pallidum. Front Immunol. 12, 759474 (2021).
Li, Q., Tong, M., Liu, L., Lin, L., Lin, Y. & Yang, T. Effect of anti-TP0136 antibodies on the progression of lesions in an infected rabbit model. Int Immunopharmacol. 83, 106428 (2020).
Chentoufi, A. A., Ulmer, J. B., BenMohamed, L. Antigen delivery platforms for Next-Generation coronavirus vaccines. Vaccines (Basel) 13, https://doi.org/10.3390/vaccines13010030 (2024).
Mantegazza, A. R., Magalhaes, J. G., Amigorena, S. & Marks, M. S. Presentation of phagocytosed antigens by MHC class I and II. Traffic 14, 135–52 (2013).
Cavazzini, D., Spagnoli, G., Mariz, F. C., Reggiani, F., Maggi, S., Franceschi, V. et al. Enhanced immunogenicity of a positively supercharged archaeon thioredoxin scaffold as a cell-penetrating antigen carrier for peptide vaccines. Front Immunol. 13, 958123 (2022).
Kiaie, S. H., Majidi, Z. N., Ahmadi, A., Bagherifar, R., Valizadeh, H., Kashanchi, F. et al. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnol. 20, 276 (2022).
Scorza, F. B., Pardi, N. New kids on the block: RNA-based influenza virus vaccines. Vaccines (Basel) 6, https://doi.org/10.3390/vaccines6020020 (2018).
Schoenmaker, L., Witzigmann, D., Kulkarni, J. A., Verbeke, R., Kersten, G., Jiskoot, W. et al. MRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 601, 120586 (2021).
Delgado, K. N., Montezuma-Rusca, J. M., Orbe, I. C., Caimano, M. J., La Vake, C. J., Luthra, A. et al. Extracellular loops of the treponema pallidum FadL orthologs TP0856 and TP0858 elicit IgG antibodies and IgG(+)-Specific B-Cells in the rabbit model of experimental syphilis. mBio 13, e163922 (2022).
Ferguson, M. R., Delgado, K. N., McBride, S., Orbe, I. C., La Vake, C. J., Caimano, M. J. et al. Use of Epivolve phage display to generate a monoclonal antibody with opsonic activity directed against a subdominant epitope on extracellular loop 4 of Treponema pallidum BamA (TP0326). Front Immunol. 14, 1222267 (2023).
Delgado, K. N., Caimano, M. J., Orbe, I. C., Vicente, C. F., La Vake, C. J., Grassmann, A. A. et al. Immunodominant extracellular loops of Treponema pallidum FadL outer membrane proteins elicit antibodies with opsonic and growth-inhibitory activities. PLoS Pathog. 20, e1012443 (2024).
Jiang, J., Xu, L., Wang, X., Wang, M., Cao, Y., Li, R. et al. A comprehensive strategy for the development of a multi-epitope vaccine targeting Treponema pallidum, utilizing heat shock proteins, encompassing the entire process from vaccine design to in vitro evaluation of immunogenicity. Front. Microbiol. 16, 1551437 (2025).
Gote, V., Bolla, P. K., Kommineni, N., Butreddy, A., Nukala, P. K. & Palakurthi, S. S. A comprehensive review of mRNA vaccines. Int. J. Mol. Sci 24, 2700 (2023).
Radolf, J. D. & Kumar, S. The Treponema pallidum Outer Membrane. Curr. Top. Microbiol Immunol. 415, 1–38 (2018).
Reid, T. B., Godornes, C., Campbell, V. L., Laing, K. J., Tantalo, L. C., Gomez, A. et al. Treponema pallidum periplasmic and membrane proteins are recognized by circulating and skin CD4+ t cells. J. Infect. Dis. 230, 281–92 (2024).
Hawley, K. L., Montezuma-Rusca, J. M., Delgado, K. N., Singh, N., Uversky, V. N., Caimano, M. J. et al. Structural modeling of the treponema pallidum outer membrane protein repertoire: a road map for deconvolution of syphilis pathogenesis and development of a syphilis vaccine. J. Bacteriol. 203, e8221 (2021).
Arroll, T. W., Centurion-Lara, A., Lukehart, S. A. & Van Voorhis, W. C. T-Cell responses to Treponema pallidum subsp. Pallidum antigens during the course of experimental syphilis infection. Infect. Immun. 67, 4757–63 (1999).
Delgado, K. N., Vicente, C. F., Hennelly, C. M., Aghakhanian, F., Parr, J. B., Claffey, K. P. et al. Development and utilization of Treponema pallidum expressing green fluorescent protein to study spirochete-host interactions and antibody-mediated clearance: Expanding the toolbox for syphilis research. mBio 16, e325324 (2025).
Sajid, A., Matias, J., Arora, G., Kurokawa, C., DePonte, K., Tang, X. et al. MRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med 13, j9827 (2021).
Pine, M., Arora, G., Hart, T. M., Bettini, E., Gaudette, B. T., Muramatsu, H. et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol. Ther. 31, 2702–14 (2023).
Lukehart, S. A., Molini, B., Gomez, A., Godornes, C., Hof, R., Fernandez, M. C. et al. Immunization with a tri-antigen syphilis vaccine significantly attenuates chancre development, reduces bacterial load, and inhibits dissemination of Treponema pallidum. Vaccine 40, 7676–92 (2022).
Rufli, T. Syphilis and HIV infection. Dermatologica 179, 113–7 (1989).
Magnuson, H. J., Thomas, E. W., Olansky, S., Kaplan, B. I., de Mello, L. & Cutler, J. C. Inoculation syphilis in human volunteers. Medicine 35, 33–82 (1956).
MacArthur, C. J. The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 120, S1–7 (2018).
Urselli, F., Gomez, A., Gray, M. D., Cameron, C. E. & Taylor, J. J. Identification of antibodies induced by immunization with the syphilis vaccine candidate Tp0751. Vaccine 50, 126804 (2025).
Acknowledgements
The authors sincerely thank Prof. Justin D Radolf and Kelly L Hawley from University of Connecticut Health for kindly providing the Pyrococcus furiosus thioredoxin (PfTrx) scaffold system and the design concept of flow cytometry experiments ensures that we can complete a more comprehensive assessment of immune activity. This work was financially supported by National Natural Science Foundation of China (82072321), Natural Science Foundation of Guangdong Province (2025A1515011165), CAMS Innovation Fund for Medical Sciences (2025-I2M-C&T-B-081), Zhujiang Scholar · Tianshan Talent Program (KDYY202023), Hospital-level Project of The First People’s Hospital of Kashi (KDYY202002), Dermatology Hospital of Southern Medical University Basic Research Nursery Program (BR202406, BR202409), European Research Council Consolidator Grant (Agreement Number 101171779, Horizon Europe Program, ERC-2024-COG funding scheme). We thank all contributors for their efforts in this study. We also express gratitude to all funding sources for their assistance and financial support.
Author information
Authors and Affiliations
Contributions
Y.J. conceived and designed the study, selected experimental methods, provided key materials, animal models, and technical platforms, and drafted the initial manuscript. N.H., L.H., X.L., G.Z., and J.L. performed experiments and collected data. X.Z., T.T., and H.L. contributed to experimental work, figure generation, and data visualization. C.L. participated in experimental procedures and project coordination. X.Zh. organized and pre-processed data. L.W. generated figures and contributed to manuscript revision and proofreading. C.A. conducted data analysis and statistical processing. B.Y. and R.C. provided scientific supervision and assisted in manuscript revision and editing. W.K. oversaw project management and scientific direction and contributed to manuscript revision, editing, and final approval. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, Y., Huang, N., Huang, L. et al. Protective immunity induced by Tp0136 epitope vaccines with mRNA LNP or protein delivery. npj Vaccines 11, 11 (2026). https://doi.org/10.1038/s41541-025-01330-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01330-7