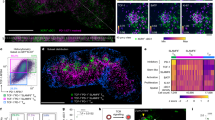
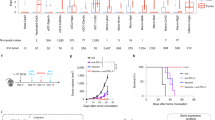
Abstract
Therapeutic cancer vaccines (TCVs) remain limited in their capacity to elicit robust CD8⁺ cytolytic T lymphocyte responses. An effective cancer vaccine adjuvant should promote expansion of antigen-specific T cells through cross-presentation by type 1 conventional dendritic cells (cDC1s). For anti-PD-1 immune checkpoint inhibitor therapy, being frequently combined with cancer vaccines, requires an expanded pool of precursor-exhausted CD8⁺ T (Tpex) cells. Here, we report Flt3L-FlaB (FB), a hybrid adjuvant that integrates FMS-like tyrosine kinase 3 ligand (Flt3L) with the TLR5 agonist flagellin B (FlaB). FB significantly expanded and activated cDC1s, accompanied by increased CD8⁺ T cells with stem-like memory (Tscm) and Tpex phenotypes in tumors and draining lymph nodes. FB-adjuvanted TCVs, combined with anti-PD-1 therapy, achieved potentiated tumor suppression and provided durable protection against metastasis and high-dose tumor rechallenge. These results establish FB as a potent TCV adjuvant with strong translational potential, particularly the combination with anti-PD-1 immune checkpoint inhibitors.

Similar content being viewed by others
Data availability
Sequence data for the engineered constructs are provided in Supplementary Table 5. All other data are included in the manuscript or are available from the corresponding authors upon reasonable request.
References
Brandenburg, A., Heine, A. & Brossart, P. Next-generation cancer vaccines and emerging immunotherapy combinations. Trends Cancer 10, 749–769 (2024).
Hollingsworth, R. E. & Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 4, 7 (2019).
Sellars, M. C., Wu, C. J. & Fritsch, E. F. Cancer vaccines: building a bridge over troubled waters. Cell 185, 2770–2788 (2022).
Saxena, M., van der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Chang, R., Gulley, J. L. & Fong, L. Vaccinating against cancer: getting to prime time. J Immunother Cancer 11, e006628 (2023).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Matusiak, M. et al. Flagellin-induced NLRC4 phosphorylation primes the inflammasome for activation by NAIP5. Proc. Natl. Acad. Sci. USA 112, 1541–1546 (2015).
Lee, S. E. et al. Flagellin is a strong vaginal adjuvant of a therapeutic vaccine for genital cancer. Oncoimmunology 5, e1081328 (2016).
Nguyen, C. T. et al. Flagellin enhances tumor-specific CD8(+) T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model. Vaccine 31, 3879–3887 (2013).
Gonzalez, C. et al. TLR5 agonists enhance anti-tumor immunity and overcome resistance to immune checkpoint therapy. Commun. Biol. 6, 31 (2023).
Lim, J. S. et al. Mucosal TLR5 activation controls healthspan and longevity. Nat. Commun. 15, 46 (2024).
Tan, W. et al. Author correction: development of an anti-tauopathy mucosal vaccine specifically targeting pathologic conformers. NPJ Vaccines 9, 121 (2024).
Puth, S. et al. An all-in-one adjuvanted therapeutic cancer vaccine targeting dendritic cell cytosol induces long-lived tumor suppression through NLRC4 inflammasome activation. Biomaterials 286, 121542 (2022).
Zheng, J. H. et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 9, eaak9537 (2017).
Pittet, M. J., Di Pilato, M., Garris, C. & Mempel, T. R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 56, 2218–2230 (2023).
Zagorulya, M. & Spranger, S. Once upon a prime: DCs shape cancer immunity. Trends Cancer 9, 172–184 (2023).
Heras-Murillo, I., Adan-Barrientos, I., Galan, M., Wculek, S. K. & Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 21, 257–277 (2024).
Giles, J. R., Globig, A. M., Kaech, S. M. & Wherry, E. J. CD8(+) T cells in the cancer-immunity cycle. Immunity 56, 2231–2253 (2023).
Moussion, C. & Delamarre, L. Antigen cross-presentation by dendritic cells: a critical axis in cancer immunotherapy. Semin. Immunol. 71, 101848 (2024).
Dahling, S. et al. Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity 55, 656–670 e658 (2022).
Schenkel, J. M. et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1(+) CD8(+) T cells in tumor-draining lymph nodes. Immunity 54, 2338–2353 e2336 (2021).
Miller, J. C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Lyman, S. D. et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75, 1157–1167 (1993).
Hegde, S. et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37, 289–307 e289 (2020).
Oba, T. et al. Overcoming primary and acquired resistance to anti-PD-L1 therapy by induction and activation of tumor-residing cDC1s. Nat. Commun. 11, 5415 (2020).
Uthaman, S. et al. Tumor microenvironment-regulating immunosenescence-independent nanostimulant synergizing with near-infrared light irradiation for antitumor immunity. ACS Appl. Mater. Interfaces 13, 4844–4852 (2021).
Tan, W. et al. A fusion protein of Derp2 allergen and flagellin suppresses experimental allergic asthma. Allergy Asthma Immunol. Res. 11, 254–266 (2019).
Puth, S. et al. A built-in adjuvant-engineered mucosal vaccine against dysbiotic periodontal diseases. Mucosal Immunol. 12, 565–579 (2019).
Lam, B. et al. In situ vaccination via tissue-targeted cDC1 expansion enhances the immunogenicity of chemoradiation and immunotherapy. J. Clin. Invest 134, e171621 (2024).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
de Sanjose, S. et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 11, 1048–1056 (2010).
Cheng, L., Wang, Y. & Du, J. Human papillomavirus vaccines: an updated review. Vaccines 8, 391 (2020).
Burmeister, C. A. et al. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res 13, 200238 (2022).
Lin, K. Y. et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 56, 21–26 (1996).
Moynihan, K. D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
Ramos da Silva, J. et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 15, eabn3464 (2023).
Gomar, C. et al. Efficacy of LCMV-based cancer immunotherapies is unleashed by intratumoral injections of polyI:C. J Immunother. Cancer 12, e008287 (2024).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 938 (2014).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723 e714 (2017).
Khim, K. et al. Deglycosylation of eukaryotic-expressed flagellin restores adjuvanticity. NPJ Vaccines 8, 139 (2023).
Brasel, K., De Smedt, T., Smith, J. L. & Maliszewski, C. R. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96, 3029–3039 (2000).
Delemarre, F. G., Hoogeveen, P. G., De Haan-Meulman, M., Simons, P. J. & Drexhage, H. A. Homotypic cluster formation of dendritic cells, a close correlate of their state of maturation. Defects in the biobreeding diabetes-prone rat. J. Leukoc. Biol. 69, 373–380 (2001).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992).
Oba, T., Kajihara, R., Yokoi, T., Repasky, E. A. & Ito, F. Neoadjuvant in situ immunomodulation enhances systemic antitumor immunity against highly metastatic tumors. Cancer Res. 81, 6183–6195 (2021).
Mayer, C. T. et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood 124, 3081–3091 (2014).
Cao, S. S. & Zhen, Y. S. Potentiation of antimetabolite antitumor activity in vivo by dipyridamole and amphotericin B. Cancer Chemother. Pharm. 24, 181–186 (1989).
Morris, M. A. et al. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur. J. Immunol. 35, 3570–3580 (2005).
Roberts, E. W. et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Shen, X. & Zhao, B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 362, k3529 (2018).
Siddiqui, I. et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211 e110 (2019).
Miller, B. C. et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+)CD38(hi) cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243 (2019).
Upadhaya, S., Neftelinov, S. T., Hodge, J. & Campbell, J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat. Rev. Drug Discov. 21, 482–483 (2022).
Xiao, W. et al. Advance in peptide-based drug development: delivery platforms, therapeutics and vaccines. Signal Transduct. Target Ther. 10, 74 (2025).
Weber, J. S. et al. Individualized neoantigen therapy mRNA-4157 (V940) plus pembrolizumab in resected melanoma: 3-year update from the mRNA-4157-P201 (KEYNOTE-942) trial. J. Clin. Oncol. 42, Lba9512–Lba9512 (2024).
Mizukoshi, E. et al. Peptide vaccine-treated, long-term surviving cancer patients harbor self-renewing tumor-specific CD8(+) T cells. Nat. Commun. 13, 3123 (2022).
Yadav, M. et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572 (2014).
Hu, Z., Ott, P. A. & Wu, C. J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18, 168–182 (2018).
Shental-Bechor, D. & Levy, Y. Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 105, 8256–8261 (2008).
Opdenakker, G., Rudd, P. M., Wormald, M., Dwek, R. A. & Van Damme, J. Cells regulate the activities of cytokines by glycosylation. FASEB J. 9, 453–457 (1995).
Lee, S. E. et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 74, 694–702 (2006).
Brown, C. C. et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 179, 846–863 e824 (2019).
Soto, J. A. et al. The role of dendritic cells during infections caused by highly prevalent viruses. Front. Immunol. 11, 1513 (2020).
Lai, J. et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 21, 914–926 (2020).
Freedman, R. S. et al. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin. Cancer Res. 9, 5228–5237 (2003).
Bhardwaj, N. et al. Flt3 ligand augments immune responses to anti-DEC-205-NY-ESO-1 vaccine through expansion of dendritic cell subsets. Nat. Cancer 1, 1204–1217 (2020).
Salmon, H. et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016).
Li, X., Liu, Y., Gui, J., Gan, L. & Xue, J. Cell identity and spatial distribution of PD-1/PD-L1 blockade responders. Adv. Sci. 11, e2400702 (2024).
Gebhardt, T., Park, S. L. & Parish, I. A. Stem-like exhausted and memory CD8(+) T cells in cancer. Nat. Rev. Cancer 23, 780–798 (2023).
Ferris, S. T. et al. cDC1 vaccines drive tumor rejection by direct presentation independently of host cDC1. Cancer Immunol. Res 10, 920–931 (2022).
D’Alise, A. M. et al. Adenoviral-based vaccine promotes neoantigen-specific CD8(+) T cell stemness and tumor rejection. Sci. Transl. Med. 14, eabo7604 (2022).
Jeon, D., Hill, E., Moseman, J. E. & McNeel, D. G. Combining toll-like receptor agonists with immune checkpoint blockade affects antitumor vaccine efficacy. J Immunother. Cancer 12, e008630 (2024).
Acknowledgements
The authors thank Dr. Sung-Woo Lee and Dr. Thanh Quang Tran for their valuable discussions. The authors also thank Min-Ju Ryu and Sun-Mi An for administrative assistance; Myeung Suk Kim for mice breeding and care; Seol Hee Hong and Yun Suhk Lee for assistance with animal experiments. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) 2020R1A5A2031185 (J.H.R), The National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) 2019R1A5A2027521 (S.E.L), The National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) RS-2025-00553055 (S.E.L) and The Korea Ministry of Food and Drug Safety grant 22213MFDS421 (S.E.L). Figures 1A, 2A, 3A, 5A, 6B, 7A, Supplementary Figs. 4, 5, 6, and 13 were generated by the authors using BioRender under an academic license.
Author information
Authors and Affiliations
Contributions
Conceptualization: G.C.D., S.E.L., J.H.R. Methodology: G.C.D., V.L., P.P., C.Y.S. Investigation and visualization: G.C.D., S.E.L., J.H.R. Supervision: S.E.L., J.H.R. Writing: G.C.D., S.E.L., J.H.R. Final approval of the version to be published: G.C.D., V.L., P.P., C.Y.S., S.E.L., J.H.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dang, G.C., Loeurng, V., Pa, P. et al. Antigen cross-presentation potentiating cancer vaccine adjuvant for T cell expansion and synergy with anti-PD-1. npj Vaccines (2026). https://doi.org/10.1038/s41541-026-01376-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-026-01376-1