Abstract
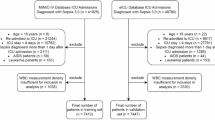
Dysregulated leukocyte responses underlie the pathobiology of sepsis, which is a leading cause of death. However, measures of leukocyte function are not routinely available in clinical care. Here we report the development and testing of an inertial microfluidic system for the label-free isolation and downstream functional assessment of leukocytes from 50 μl of peripheral blood. We used the system to assess leukocyte phenotype and function in serial samples from 18 hospitalized patients with sepsis and 10 healthy subjects. The sepsis samples had significantly higher levels of CD16dim and CD16− neutrophils and CD16+ ‘intermediate’ monocytes, as well as significantly lower levels of neutrophil-elastase release, O2− production and phagolysosome formation. Repeated sampling of sepsis patients over 7 days showed that leukocyte activation (measured by isodielectric separation) and leukocyte phenotype and function were significantly more predictive of the clinical course than complete-blood-count parameters. We conclude that the serial assessment of leukocyte function in microlitre blood volumes is feasible and that it provides significantly more prognostic information than leukocyte counting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the results in this study are available in the Article and Supplementary Information. The raw patient data are available from the authors, subject to approval from the Institutional Review Board of Partner’s Healthcare.
Code availability
The custom code used in this study is available at https://github.com/bdlevylab/BDLevy-Lab.
References
Delano, M. J. & Ward, P. A. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J. Clin. Invest. 126, 23–31 (2016).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851 (2013).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014).
Majno, G. & Joris, I. Cells, Tissues, and Disease 2nd edn (Oxford University Press, 2004).
Spite, M. et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 (2009).
Singer, M. et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). J. Am. Med. Assoc. 315, 801–810 (2016).
Rhee, C. et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. J. Am. Med. Assoc. 318, 1241–1249 (2017).
Buchegger, P. & Preininger, C. Point-of-care chip for diagnosis of sepsis: joining biomarker quantification and bacterial class identification. Biomed. Tech. https://doi.org/10.1515/bmt-2013-4146 (2013).
Hassan, U. et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat. Commun. 8, 15949 (2017).
Kemmler, M., Sauer, U., Schleicher, E., Preininger, E. & Brandenburg, A. Biochip point-of-care device for sepsis diagnostics. Sens. Actuat. B 192, 205–215 (2014).
Reddy, B. et al. Point-of-care sensors for the management of sepsis. Nat. Biomed. Eng. 2, 640–648 (2018).
Zhang, Y. et al. Detection of sepsis in patient blood samples using CD64 expression in a microfluidic cell separation device. Analyst 143, 241–249 (2017).
Zhang, Y. et al. Multiparameter affinity microchip for early sepsis diagnosis based on CD64 and CD69 expression and cell capture. Anal. Chem. 90, 7204–7211 (2018).
Zhou, Y. et al. Detection of culture-negative sepsis in clinical blood samples using a microfluidic assay for combined CD64 and CD69 cell capture. Anal. Chim. Acta 1062, 110–117 (2019).
Ellett, F. et al. Diagnosis of sepsis from a drop of blood by measurement of spontaneous neutrophil motility in a microfluidic assay. Nat. Biomed. Eng. 2, 207–214 (2018).
Vincent, J. L. et al. Anemia and blood transfusion in critically ill patients. J. Am. Med. Assoc. 288, 1499–1507 (2002).
Ryu, H.et al. Label-free neutrophil enrichment from patient-derived airway secretion using closed-loop inertial microfluidics. J. Vis. Exp. https://doi.org/10.3791/57673 (2018).
Wu, L., Guan, G., Hou, H. W., Bhagat, A. A. & Han, J. Separation of leukocytes from blood using spiral channel with trapezoid cross-section. Anal. Chem. 84, 9324–9331 (2012).
Ryu, H. et al. Patient-derived airway secretion dissociation technique to isolate and concentrate immune cells using closed-loop inertial microfluidics. Anal. Chem. 89, 5549–5556 (2017).
Amini, H., Lee, W. & Di Carlo, D. Inertial microfluidic physics. Lab Chip 14, 2739–2761 (2014).
Di Carlo, D. Inertial microfluidics. Lab Chip 9, 3038–3046 (2009).
Prieto, J. L. et al. Monitoring sepsis using electrical cell profiling. Lab Chip 16, 4333–4340 (2016).
Gonzalez, I. et al. A label free disposable device for rapid isolation of rare tumor cells from blood by ultrasounds. Micromachines 9, 129 (2018).
Li, P. et al. Acoustic separation of circulating tumor cells. Proc. Natl Acad. Sci. USA 112, 4970–4975 (2015).
Mach, A. J. & Di Carlo, D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol. Bioeng. 107, 302–311 (2010).
Nivedita, N. & Papautsky, I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 7, 54101 (2013).
Smith, A. J. et al. Rapid cell separation with minimal manipulation for autologous cell therapies. Sci. Rep. 7, 41872 (2017).
Ai, Y., Sanders, C. K. & Marrone, B. L. Separation of Escherichia coli bacteria from peripheral blood mononuclear cells using standing surface acoustic waves. Anal. Chem. 85, 9126–9134 (2013).
Wu, M. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl Acad. Sci. USA 114, 10584–10589 (2017).
Vykoukal, J., Vykoukal, D. M., Freyberg, S., Alt, E. U. & Gascoyne, P. R. Enrichment of putative stem cells from adipose tissue using dielectrophoretic field-flow fractionation. Lab Chip 8, 1386–1393 (2008).
Vahey, M. D., Quiros Pesudo, L., Svensson, J. P., Samson, L. D. & Voldman, J. Microfluidic genome-wide profiling of intrinsic electrical properties in Saccharomyces cerevisiae. Lab Chip 13, 2754–2763 (2013).
Di Carlo, D., Irimia, D., Tompkins, R. G. & Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl Acad. Sci. USA 104, 18892–18897 (2007).
Nivedita, N., Ligrani, P. & Papautsky, I. Dean flow dynamics in low-aspect ratio spiral microchannels. Sci. Rep. 7, 44072 (2017).
Wang, X. & Papautsky, I. Size-based microfluidic multimodal microparticle sorter. Lab Chip 15, 1350–1359 (2015).
Hou, H. W. et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 3, 1259 (2013).
Khoo, B. L. et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS ONE 9, e99409 (2014).
Warkiani, M. E. et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 14, 128–137 (2014).
Warkiani, M. E. et al. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 139, 3245–3255 (2014).
Warkiani, M. E. et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 11, 134–148 (2016).
Yin, L. et al. Microfluidic label-free selection of mesenchymal stem cell subpopulation during culture expansion extends the chondrogenic potential in vitro. Lab Chip 18, 878–889 (2018).
Choi, K. et al. Negative selection by spiral inertial microfluidics improves viral recovery and sequencing from blood. Anal. Chem. 90, 4657–4662 (2018).
Hou, H. W., Bhattacharyya, R. P., Hung, D. T. & Han, J. Direct detection and drug-resistance profiling of bacteremias using inertial microfluidics. Lab Chip 15, 2297–2307 (2015).
Abdulla, A., Liu, W., Gholamipour-Shirazi, A., Sun, J. & Ding, X. High-throughput isolation of circulating tumor cells using cascaded inertial focusing microfluidic channel. Anal. Chem. 90, 4397–4405 (2018).
Nivedita, N., Garg, N., Lee, A. P. & Papautsky, I. A high throughput microfluidic platform for size-selective enrichment of cell populations in tissue and blood samples. Analyst 142, 2558–2569 (2017).
Shen, X. F., Cao, K., Jiang, J. P., Guan, W. X. & Du, J. F. Neutrophil dysregulation during sepsis: an overview and update. J. Cell. Mol. Med. 21, 1687–1697 (2017).
Solomkin, J. S., Cotta, L. A., Brodt, J. K. & Hurst, J. M. Regulation of neutrophil superoxide production in sepsis. Arch. Surg. 120, 93–98 (1985).
Pillay, J. et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Invest. 122, 327–336 (2012).
Lollike, K. & Lindau, M. Membrane capacitance techniques to monitor granule exocytosis in neutrophils. J. Immunol. Methods 232, 111–120 (1999).
Griffith, A. W. & Cooper, J. M. Single-cell measurements of human neutrophil activation using electrorotation. Anal. Chem. 70, 2607–2612 (1998).
Holmes, D. et al. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip 9, 2881–2889 (2009).
Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell. Immunol. 289, 135–139 (2014).
Abdulnour, R. E. et al. Early intravascular events are associated with development of acute respiratory distress syndrome. A substudy of the LIPS-A clinical trial. Am. J. Respir. Crit. Care Med. 197, 1575–1585 (2018).
Patel, A. A. et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923 (2017).
Zawada, A. M. et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 118, e50–e61 (2011).
Pierrakos, C. & Vincent, J. L. Sepsis biomarkers: a review. Crit. Care 14, R15 (2010).
Villani, A. C. et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017).
Seymour, C. W. Assessment of clinical criteria for sepsis. JAMA 315, 762 (2016).
Dolinay, T. et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 185, 1225–1234 (2012).
Fredenburgh, L. E. et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight 3, e124039 (2018).
Barnig, C. et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 5, 174ra126 (2013).
Neutrophil Elastase Activity Assay Kit. Cayman Chemical https://www.caymanchem.com/product/600610/neutrophil-elastase-activity-assay-kit (2019).
Acknowledgements
The authors acknowledge the contributions of G. Zhu, L. A. Cosimi and additional members of the Brigham and Women’s Registry of Critical Illness (including L. Fredenburgh, P. Dieffenbach, S. Ash and J. Englert). The work was supported by grant nos. U24-AI118656 (B.D.L., J.V.), K08-HL130540 (R.E.A.) and K12-HD047349 (M.G.D).
Author information
Authors and Affiliations
Contributions
B.J., H.R. and D.-H.L. contributed equally to this study. J.H., J.V. and B.D.L. coconceived the study. B.J., H.R., D.-H.L., R.E.A., B.D.E., M.G.D., J.L., R.M.B., N.K., J.H., J.V. and B.D.L. designed the experiments and interpreted the results. B.J., D.-H.L., R.E.A., B.D.E, J.L., J.V. and B.D.L. performed and analysed the functional biological experiments. H.R. and J.H. designed and performed experiments using the inertial microfluidic system. A.H., M.P.-V., M.E.B. and R.M.B. provided blood samples and clinical data from patients with sepsis. The manuscript was written by B.J., H.R., D.-H.L., R.E.A., B.D.E., M.G.D., J.L., N.K., R.M.B., J.H., J.V. and B.D.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Rights and permissions
About this article
Cite this article
Jundi, B., Ryu, H., Lee, DH. et al. Leukocyte function assessed via serial microlitre sampling of peripheral blood from sepsis patients correlates with disease severity. Nat Biomed Eng 3, 961–973 (2019). https://doi.org/10.1038/s41551-019-0473-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-019-0473-5
This article is cited by
-
Leveraging laboratory biomarkers to predict urosepsis after upper urinary tract stone surgery: an explainable machine learning approach
BMC Medical Informatics and Decision Making (2025)
-
Whole blood biophysical immune profiling of newborn infants correlates with immune responses
Pediatric Research (2025)
-
Separation of mononuclear cells from progenitor products by a novel inertial microfluidic method
Biomedical Microdevices (2025)
-
Single-cell electro-mechanical shear flow deformability cytometry
Microsystems & Nanoengineering (2024)
-
Exosomal Drug Delivery Systems: A Novel Therapy Targeting PD-1 in Septic-ALI
Stem Cell Reviews and Reports (2024)