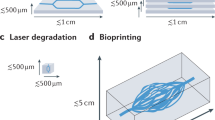
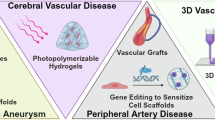
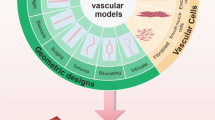
Abstract
The loss of organ function following traumatic injury is often irreversible and the demand for organ replacements continues to exceed supply. This discrepancy has driven the development of therapies and engineered tissues for the repair or replacement of damaged tissues. However, the survival of engineered tissues is constrained by the challenge of establishing a functional vasculature. Efforts have therefore focused on strategies that induce vascularization in tissue implants or stimulate vascular growth in recipients of the therapies. Here we discuss recent advances in vascular biology, biomaterials chemistry and 3D printing techniques for vascular patterning in engineered tissues. For tissue regeneration to be clinically viable, vascular formation must be guided across scales ranging from micrometres to millimetres through biological, chemical and physical approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brittberg, M., Recker, D., Ilgenfritz, J. & Saris, D. B. F. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am. J. Sports Med. 46, 1343–1351 (2018).
Schmidt, C. Gintuit cell therapy approval signals shift at US regulator. Nat. Biotechnol. 30, 479–479 (2012).
Swan, M. Steady advance of stem cell therapies: report from the 2011 World Stem Cell Summit, Pasadena, California, October 3–5. Rejuvenation Res. 14, 699–704 (2011).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Phelps, E. A. & Garcia, A. J. Update on therapeutic vascularization strategies. Regen. Med. 4, 65–80 (2009).
Gandhi, J. K., Opara, E. C. & Brey, E. M. Alginate-based strategies for therapeutic vascularization. Ther. Deliv. 4, 327–341 (2013).
Bowers, D. T., Song, W., Wang, L.-H. & Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 95, 131–151 (2019).
Gianni‐Barrera, R. et al. Therapeutic vascularization in regenerative medicine. Stem Cells Transl. Med. 9, 433–444 (2020).
Wilson, Z. E. et al. Inter-individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br. J. Clin. Pharmacol. 56, 433–440 (2003).
Risau, W. & Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 11, 73–91 (1995).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674 (1997).
Zhang, B. et al. Microfabrication of AngioChip, a biodegradable polymer scaffold with microfluidic vasculature. Nat. Protoc. 13, 1793–1813 (2018).
Zhang, B. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 15, 669–678 (2016).
Szklanny, A. A. et al. 3D bioprinting of engineered tissue flaps with hierarchical vessel networks (VesselNet) for direct host-to-implant perfusion. Adv. Mater. 33, 2102661 (2021).
Wang, X. et al. Improving islet engraftment by gene therapy. J. Transplant. 2011, e594851 (2011).
Ajioka, I. et al. Establishment of heterotropic liver tissue mass with direct link to the host liver following implantation of hepatocytes transfected with vascular endothelial growth factor gene in mice. Tissue Eng. 7, 335–344 (2001).
Vlahos, A. E. et al. Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy. Biomaterials 232, 119710 (2020).
Weaver, J. D. et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 3, e1700184 (2017).
Phelps, E. A., Templeman, K. L., Thulé, P. M. & García, A. J. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl. Res. 5, 125–136 (2015).
Mirabella, T. et al. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng. 1, 0083 (2017).
Baranski, J. D. et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl Acad. Sci. USA 110, 7586–7591 (2013).
Song, W. et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 10, 4602 (2019).
Mastrullo, V., Cathery, W., Velliou, E., Madeddu, P. & Campagnolo, P. Angiogenesis in tissue engineering: as nature intended? Front. Bioeng. Biotechnol. 2020, 188 (2020).
Goldie, L. C., Nix, M. K. & Hirschi, K. K. Embryonic vasculogenesis and hematopoietic specification. Organogenesis 4, 257–263 (2008).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–966 (1997).
Asahara, T. et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18, 3964–3972 (1999).
Takahashi, T. et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 (1999).
De Spiegelaere, W. et al. Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. J. Vasc. Res. 49, 390–404 (2012).
Rouwkema, J. & Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 34, 733–745 (2016).
Burri, P. H., Hlushchuk, R. & Djonov, V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev. Dyn. 231, 474–488 (2004).
Hellström, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Roesel, J. F. & Nanney, L. B. Assessment of differential cytokine effects on angiogenesis using an in vivo model of cutaneous wound repair. J. Surg. Res. 58, 449–459 (1995).
Wei, L.-H. et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22, 1517–1527 (2003).
Li, A., Dubey, S., Varney, M. L., Dave, B. J. & Singh, R. K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 170, 3369–3376 (2003).
Voronov, E. et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl Acad. Sci. USA 100, 2645–2650 (2003).
Miller, J. S. et al. Bioactive hydrogels made from step-growth derived PEG–peptide macromers. Biomaterials 31, 3736–3743 (2010).
Djonov, V., Baum, O. & Burri, P. H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 314, 107–117 (2003).
Caduff, J. H., Fischer, L. C. & Burri, P. H. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat. Rec. 216, 154–164 (1986).
Grundmann, S., Piek, J. J., Pasterkamp, G. & Hoefer, I. E. Arteriogenesis: basic mechanisms and therapeutic stimulation. Eur. J. Clin. Invest. 37, 755–766 (2007).
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395 (2000).
Giacca, M. & Zacchigna, S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 19, 622–629 (2012).
Henry, T. D. et al. The VIVA trial. Circulation 107, 1359–1365 (2003).
Simón-Yarza, T. et al. Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Theranostics 2, 541–552 (2012).
Darden, J., Payne, L. B., Zhao, H. & Chappell, J. C. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis 22, 167–183 (2018).
Blatchley, M. R., Hall, F., Wang, S., Pruitt, H. C. & Gerecht, S. Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis. Sci. Adv. 5, eaau7518 (2019). This study describes the mechanism through which hypoxia and the material properties of the matrix impact vasculogenesis.
Longchamp, A. et al. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 173, 117–129.e14 (2018).
Fiedler, J. et al. Functional microRNA library screening identifies the hypoxaMiR MiR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid. Redox Signal. 21, 1167–1176 (2014).
Dou, C. et al. Graphene-based microRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv. Sci. 5, 1700578 (2018).
Li, Y. et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 7, eabd6740 (2021).
Lamichhane, T. N., Leung, C. A., Douti, L. Y. & Jay, S. M. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of microRNAs and long non-coding RNAs. Sci. Rep. 7, 13794 (2017).
Welten, S. M. J. et al. Inhibition of 14q32 microRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ. Res. 115, 696–708 (2014).
Liu, B. et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2, 293–303 (2018).
Kawamoto, A. et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107, 461–468 (2003).
Losordo, D. W. et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina. Circulation 115, 3165–3172 (2007).
Calderon, G. A. et al. Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate gels. Biomater. Sci. 5, 1652–1660 (2017).
Moon, J. J. et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 31, 3840–3847 (2010).
Pedowitz, N. J., Batt, A. R., Darabedian, N. & Pratt, M. R. MYPT1 O-GlcNAc modification regulates sphingosine-1-phosphate mediated contraction. Nat. Chem. Biol. 17, 169–177 (2021). This study details how a particular post-translational modification affects diabetic wound healing.
Nadkarni, S. et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl Acad. Sci. USA 113, E8415–E8424 (2016).
Mor, F., Quintana, F. J. & Cohen, I. R. Angiogenesis–inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J. Immunol. 172, 4618–4623 (2004).
Marek, N. et al. Increased spontaneous production of VEGF by CD4+ T cells in type 1 diabetes. Clin. Immunol. 137, 261–270 (2010).
Ribatti, D. & Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 13, 2822–2833 (2009).
Graney, P. L. et al. Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci. Adv. 6, eaay6391 (2020).
Fleischer, S., Tavakol, D. N. & Vunjak-Novakovic, G. From arteries to capillaries: approaches to engineering human vasculature. Adv. Funct. Mater. 30, 1910811 (2020).
Zhao, S. et al. Application of stem cells in engineered vascular graft and vascularized organs. Semin. Cell Dev. Biol. 144, 31–40 (2023).
Kumar, A. H. S. & Caplice, N. M. Clinical potential of adult vascular progenitor cells. Arter. Thromb. Vasc. Biol. 30, 1080–1087 (2010).
Bayraktutan, U. Endothelial progenitor cells: potential novel therapeutics for ischaemic stroke. Pharmacol. Res. 144, 181–191 (2019).
Nowbar, A. N. et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. Br. Med. J. 348, g2688 (2014).
Krawiec, J. T. & Vorp, D. A. Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials 33, 3388–3400 (2012).
Galat, V. et al. Transgene reactivation in induced pluripotent stem cell derivatives and reversion to pluripotency of induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Dev. 25, 1060–1072 (2016).
Hentze, H. et al. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2, 198–210 (2009).
Quint, C. et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl Acad. Sci. USA 108, 9214–9219 (2011).
Neff, L. P. et al. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J. Vasc. Surg. 53, 426–434 (2011).
Kaushal, S. et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 7, 1035–1040 (2001).
He, H., Shirota, T., Yasui, H. & Matsuda, T. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J. Thorac. Cardiovasc. Surg. 126, 455–464 (2003).
Zhu, C. et al. Development of anti-atherosclerotic tissue-engineered blood vessel by A20-regulated endothelial progenitor cells seeding decellularized vascular matrix. Biomaterials 29, 2628–2636 (2008).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Bu, L. et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 460, 113–117 (2009).
Ferreira, L. S. et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ. Res. 101, 286–294 (2007).
Levenberg, S., Ferreira, L. S., Chen-Konak, L., Kraehenbuehl, T. P. & Langer, R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat. Protoc. 5, 1115–1126 (2010).
Hill, K. L. et al. Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. Exp. Hematol. 38, 246–257.e1 (2010).
Levenberg, S., Golub, J. S., Amit, M., Itskovitz-Eldor, J. & Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 4391–4396 (2002).
Cheung, C. & Sinha, S. Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. J. Mol. Cell. Cardiol. 51, 651–664 (2011).
Sone, M. et al. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arter. Thromb. Vasc. Biol. 27, 2127–2134 (2007).
Taura, D. et al. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler. Thromb. Vasc. Biol. 29, 1100–1103 (2009).
Patsch, C. et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 17, 994–1003 (2015).
Sundaram, S., Echter, A., Sivarapatna, A., Qiu, C. & Niklason, L. Small-diameter vascular graft engineered using human embryonic stem cell-derived mesenchymal cells. Tissue Eng. A 20, 740–750 (2014).
Wang, L. et al. Fabrication of tissue-engineered vascular grafts with stem cells and stem cell-derived vascular cells. Expert Opin. Biol. Ther. 16, 317–330 (2016).
Jang, S., Collin de l’Hortet, A. & Soto-Gutierrez, A. Induced pluripotent stem cell-derived endothelial cells: overview, current advances, applications, and future directions. Am. J. Pathol. 189, 502–512 (2019).
Margariti, A. et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc. Natl Acad. Sci. USA 109, 13793–13798 (2012).
Shen, M., Quertermous, T., Fischbein, M. P. & Wu, J. C. Generation of vascular smooth muscle cells from induced pluripotent stem cells: methods, applications, and considerations. Circ. Res. 128, 670–686 (2021).
Generali, M. et al. Autologous endothelialized small-caliber vascular grafts engineered from blood-derived induced pluripotent stem cells. Acta Biomater. 97, 333–343 (2019).
Abaci, H. E. et al. Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv. Healthc. Mater. 5, 1800–1807 (2016).
Ciampi, O. et al. Engineering the vasculature of decellularized rat kidney scaffolds using human induced pluripotent stem cell-derived endothelial cells. Sci. Rep. 9, 8001 (2019).
Wang, Y. Y. et al. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials 35, 8960–8969 (2014).
Luo, J. et al. Tissue-engineered vascular grafts with advanced mechanical strength from human iPSCs. Cell Stem Cell 26, 251–261.e8 (2020).
Gui, L. et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 102, 120–129 (2016).
Atchison, L. et al. iPSC-derived endothelial cells affect vascular function in a tissue-engineered blood vessel model of Hutchinson–Gilford progeria syndrome. Stem Cell Rep. 14, 325–337 (2020).
Owens, G. K., Kumar, M. S. & Wamhoff, B. R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 (2004).
Majesky, M. W. Developmental basis of vascular smooth muscle diversity. Arter. Thromb. Vasc. Biol. 27, 1248–1258 (2007).
Turner, M. et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 13, 382–384 (2013).
Wilmut, I. et al. Development of a global network of induced pluripotent stem cell haplobanks. Regen. Med. 10, 235–238 (2015).
Lee, S. et al. Repurposing the cord blood bank for haplobanking of HLA-homozygous iPSCs and their usefulness to multiple populations. Stem Cells 36, 1552–1566 (2018).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 42, 413–423 (2024).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772 (2017).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Neve, A., Cantatore, F. P., Maruotti, N., Corrado, A. & Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed. Res. Int. 2014, 756078 (2014).
Senger, D. R. & Davis, G. E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 3, a005090 (2011).
Stupack, D. G. & Cheresh, D. A. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci. STKE 2002, pe7 (2002).
Ouyang, L. et al. MMP‑sensitive PEG hydrogel modified with RGD promotes bFGF, VEGF and EPC‑mediated angiogenesis. Exp. Ther. Med. 18, 2933–2941 (2019).
Nemati, S. et al. Alginate–gelatin encapsulation of human endothelial cells promoted angiogenesis in in vivo and in vitro milieu. Biotechnol. Bioeng. 114, 2920–2930 (2017).
Li, Z. et al. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 13, 88–100 (2015).
Seo, Y., Jung, Y. & Kim, S. H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 67, 270–281 (2018).
Griffin, M. E., Sorum, A. W., Miller, G. M., Goddard, W. A. & Hsieh-Wilson, L. C. Sulfated glycans engage the Ang–Tie pathway to regulate vascular development. Nat. Chem. Biol. 17, 178–186 (2021).
Jia, J. et al. Evolutionarily conserved sequence motif analysis guides development of chemically defined hydrogels for therapeutic vascularization. Sci. Adv. 6, eaaz5894 (2020).
Ruehle, M. A. et al. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci. Adv. 6, eabb6351 (2020).
Mandrycky, C., Hadland, B. & Zheng, Y. 3D curvature-instructed endothelial flow response and tissue vascularization. Sci. Adv. 6, eabb3629 (2020). This study quantifies the effect of vessel geometry and perfusion-related forces on endothelial cells.
Lee, J. et al. Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration. Nat. Biomed. Eng. 5, 89–102 (2021).
Wolf, K. J., Weiss, J. D., Uzel, S. G. M., Skylar-Scott, M. A. & Lewis, J. A. Biomanufacturing human tissues via organ building blocks. Cell Stem Cell 29, 667–677 (2022).
Munarin, F., Kant, R. J., Rupert, C. E., Khoo, A. & Coulombe, K. L. K. Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 251, 120033 (2020).
Nih, L. R., Gojgini, S., Carmichael, S. T. & Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 17, 642–651 (2018).
Lee, A. S. et al. Prolonged survival of transplanted stem cells after ischaemic injury via the slow release of pro-survival peptides from a collagen matrix. Nat. Biomed. Eng. 2, 104–113 (2018).
Aday, S. et al. Synthetic microparticles conjugated with VEGF165 improve the survival of endothelial progenitor cells via microRNA-17 inhibition. Nat. Commun. 8, 747 (2017).
Coindre, V. F., Carleton, M. M. & Sefton, M. V. Methacrylic acid copolymer coating enhances constructive remodeling of polypropylene mesh by increasing the vascular response. Adv. Healthc. Mater. 8, 1900667 (2019).
Coindre, V. F., Kinney, S. M. & Sefton, M. V. Methacrylic acid copolymer coating of polypropylene mesh chamber improves subcutaneous islet engraftment. Biomaterials 259, 120324 (2020).
Yu, Y. et al. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 7, eabd8217 (2021).
Li, X. et al. Nanofiber-hydrogel composite-mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 11, eaau6210 (2019).
Mazio, C. et al. Pre-vascularized dermis model for fast and functional anastomosis with host vasculature. Biomaterials 192, 159–170 (2019).
Roberts, S. et al. Injectable tissue integrating networks from recombinant polypeptides with tunable order. Nat. Mater. 17, 1154–1163 (2018). This study describes the creation of partially ordered peptides for vascularizing materials with tunable properties on the basis of ordered/disordered domains.
Wang, L. et al. Development of a centrally vascularized tissue engineering bone graft with the unique core-shell composite structure for large femoral bone defect treatment. Biomaterials 175, 44–60 (2018).
Dor, Y. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 21, 1939–1947 (2002).
Tafuro, S. et al. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc. Res. 83, 663–671 (2009).
Ozawa, C. R. et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest. 113, 516–527 (2004).
Ozaki, H. et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood–retinal barrier in rabbits and primates. Exp. Eye Res. 64, 505–517 (1997).
Murphy, W. L., Peters, M. C., Kohn, D. H. & Mooney, D. J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 21, 2521–2527 (2000).
Lee, K. W. et al. Sustained release of vascular endothelial growth factor from calcium-induced alginate hydrogels reinforced by heparin and chitosan. Transplant. Proc. 36, 2464–2465 (2004).
Zisch, A. H. et al. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 17, 2260–2262 (2003).
Seliktar, D., Zisch, A. H., Lutolf, M. P., Wrana, J. L. & Hubbell, J. A. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J. Biomed. Mater. Res. A 68, 704–716 (2004).
Zisch, A. H., Schenk, U., Schense, J. C., Sakiyama-Elbert, S. E. & Hubbell, J. A. Covalently conjugated VEGF–fibrin matrices for endothelialization. J. Control. Release 72, 101–113 (2001).
Moulisová, V. et al. Engineered microenvironments for synergistic VEGF–integrin signalling during vascularization. Biomaterials 126, 61–74 (2017).
Lee, T. T. et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 14, 352–360 (2015).
Brady, A. C. et al. Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue Eng. A 19, 2544–2552 (2013).
Liang, J. P., Accolla, R. P., Jiang, K., Li, Y. & Stabler, C. L. Controlled release of anti-inflammatory and proangiogenic factors from macroporous scaffolds. Tissue Eng. A 27, 1275–1289 (2021).
Chen, T. T. et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595–609 (2010).
Yin, N. et al. VEGF-conjugated alginate hydrogel prompt angiogenesis and improve pancreatic islet engraftment and function in type 1 diabetes. Mater. Sci. Eng. C 59, 958–964 (2016).
Lee, J., Yang, C., Ahn, S., Choi, Y. & Lee, K. Enhanced NO-induced angiogenesis via NO/H2S co-delivery from self-assembled nanoparticles. Biomater. Sci. 9, 5150–5159 (2021).
Santulli, G. et al. In vivo properties of the proangiogenic peptide QK. J. Transl. Med. 7, 41 (2009).
D’Andrea, L. D. et al. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc. Natl Acad. Sci. USA 102, 14215–14220 (2005). This study describes the creation of a VEGF mimic with vascularizing properties similar to those of the parent protein.
Kumar, V. A. et al. Highly angiogenic peptide nanofibers. ACS Nano 9, 860–868 (2015). This study describes the creation of an angiogenic self-assembling peptide–hydrogel with a VEGF mimic in the primary sequence.
Kumar, V. A. et al. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials 98, 113–119 (2016).
Moore, A. N. et al. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 161, 154–163 (2018).
Lopez-Silva, T. L. et al. Chemical functionality of multidomain peptide hydrogels governs early host immune response. Biomaterials 231, 119667 (2020).
Carrejo, N. C. et al. Multidomain peptide hydrogel accelerates healing of full-thickness wounds in diabetic mice. ACS Biomater. Sci. Eng. 4, 1386–1396 (2018).
Lopez-Silva, T. L. et al. Self-assembling multidomain peptide hydrogels accelerate peripheral nerve regeneration after crush injury. Biomaterials 265, 120401 (2021).
Lai, C. S. E. et al. A combined conduit-bioactive hydrogel approach for regeneration of transected sciatic nerves. ACS Appl. Bio Mater. 2022, 4611–4624 (2022).
Howard, D., Buttery, L. D., Shakesheff, K. M. & Roberts, S. J.Tissue engineering: strategies, stem cells and scaffolds. J. Anat. 213, 66–72 (2008).
Kinstlinger, I. S. & Miller, J. S. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip 16, 2025–2043 (2016).
Sarker, M. D., Naghieh, S., Sharma, N. K. & Chen, X. 3D biofabrication of vascular networks for tissue regeneration: a report on recent advances. J. Pharm. Anal. 8, 277–296 (2018).
Devillard, C. D. & Marquette, C. A. Vascular tissue engineering: challenges and requirements for an ideal large scale blood vessel. Front. Bioeng. Biotechnol. 9, 721843 (2021).
Hynes, W. F. et al. Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci. Adv. 6, eabb3308 (2020).
Kinstlinger, I. S. et al. Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates. Nat. Biomed. Eng. 4, 916–932 (2020).
Papaioannou, T. G. & Stefanadis, C. Vascular wall shear stress: basic principles and methods. Hell. J. Cardiol. 46, 9–15 (2005).
Yilmaz, B., Al Rashid, A., Mou, Y. A., Evis, Z. & Koç, M. Bioprinting: a review of processes, materials and applications. Bioprinting 23, e00148 (2021).
Emerson, A. E., McCall, A. B., Brady, S. R., Slaby, E. M. & Weaver, J. D. Hydrogel injection molding to generate complex cell encapsulation geometries. ACS Biomater. Sci. Eng. 8, 4002–4013 (2022).
Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019). This study describes a method for rapidly creating vascular networks between densely packed cellular aggregates, for tissue engineering applications.
Lee, A. et al. 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 (2019). Fabricates large-scale tissue engineering scaffolds using extrusion 3D printing.
Grigoryan, B. et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 (2019). This study describes the use of 3D printing to fabricate high-resolution structures using biocompatible materials.
Kelly, B. E. et al. Volumetric additive manufacturing via tomographic reconstruction. Science 363, 1075–1079 (2019).
Regehly, M. et al. Xolography for linear volumetric 3D printing. Nature 588, 620–624 (2020).
Anandakrishnan, N. et al. Fast stereolithography printing of large-scale biocompatible hydrogel models. Adv. Healthc. Mater. 10, 2002103 (2021).
Galarraga, J. H., Kwon, M. Y. & Burdick, J. A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 9, 19987 (2019).
Ouyang, L., Highley, C. B., Sun, W. & Burdick, J. A. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv. Mater. 29, 1604983 (2017).
Datta, P., Dey, M., Ataie, Z., Unutmaz, D. & Ozbolat, I. T. 3D bioprinting for reconstituting the cancer microenvironment. npj Precis. Oncol. 4, 18 (2020).
Ma, X. et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl Acad. Sci. USA 113, 2206–2211 (2016).
Skylar-Scott, M. A., Mueller, J., Visser, C. W. & Lewis, J. A. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575, 330–335 (2019).
Shiwarski, D. J., Hudson, A. R., Tashman, J. W. & Feinberg, A. W. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 5, 010904 (2021).
Gao, G. et al. Construction of a novel in vitro atherosclerotic model from geometry-tunable artery equivalents engineered via in-bath coaxial cell printing. Adv. Funct. Mater. 31, 2008878 (2021).
Cho, W. W., Ahn, M., Kim, B. S. & Cho, D. W. Blood-lymphatic integrated system with heterogeneous melanoma spheroids via in-bath three-dimensional bioprinting for modelling of combinational targeted therapy. Adv. Sci. 9, e2202093 (2022).
Kim, B. S. et al. Construction of tissue-level cancer-vascular model with high-precision position control via in situ 3D cell printing. Small Methods 5, 2100072 (2021).
Singh, N. K. et al. Coaxial cell printing of a human glomerular model: anin vitroglomerular filtration barrier and its pathophysiology. Biofabrication 15, 024101 (2023).
Boularaoui, S., Al Hussein, G., Khan, K. A., Christoforou, N. & Stefanini, C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 20, e00093 (2020).
Wang, Z. et al. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 7, 045009 (2015).
Derakhshanfar, S. et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 3, 144–156 (2018).
Hwang, H. H., Zhu, W., Victorine, G., Lawrence, N. & Chen, S. 3D-printing of functional biomedical microdevices via light- and extrusion-based approaches. Small Methods 2, 1700277 (2018).
Ikuta, K. & Hirowatari, K. Real three dimensional micro fabrication using stereo lithography and metal molding. In Proc. IEEE Micro Electro Mechanical Systems 42–47 (IEEE, 2002).
Fonseca, A. C. et al. Emulating human tissues and organs: a bioprinting perspective toward personalized medicine. Chem. Rev. 120, 11093–11139 (2020).
Warner, J., Soman, P., Zhu, W., Tom, M. & Chen, S. Design and 3D printing of hydrogel scaffolds with fractal geometries. ACS Biomater. Sci. Eng. 2, 1763–1770 (2016).
Zhu, W. et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 124, 106–115 (2017).
Tomov, M. L. et al. A 3D bioprinted in vitro model of pulmonary artery atresia to evaluate endothelial cell response to microenvironment. Adv. Healthc. Mater. 10, 2100968 (2021).
Bagheri Saed, A. et al. An in vitro study on the key features of poly l-lactic acid/biphasic calcium phosphate scaffolds fabricated via DLP 3D printing for bone grafting. Eur. Polym. J. 141, 110057 (2020).
Jiang, P. et al. Grayscale stereolithography of gradient hydrogel with site-selective shape deformation. Adv. Mater. Technol. 7, 2101288 (2022).
You, S. et al. Mitigating scattering effects in light-based three-dimensional printing using machine learning. J. Manuf. Sci. Eng. 142, 081002 (2020).
Bernal, P. N. et al. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv. Mater. 34, 2110054 (2022).
You, S. et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 9, eade7923 (2023).
Seymour, A. J., Westerfield, A. D., Cornelius, V. C., Skylar-Scott, M. A. & Heilshorn, S. C. Bioprinted microvasculature: progressing from structure to function. Biofabrication 14, 022002 (2022). This review provides a comprehensive overview of the challenges and strategies surrounding the 3D printing of vasculature.
Urciuolo, A. et al. Hydrogel-in-hydrogel live bioprinting for guidance and control of organoids and organotypic cultures. Nat. Commun. 14, 3128 (2023).
Betz, C., Lenard, A., Belting, H. G. & Affolter, M. Cell behaviors and dynamics during angiogenesis. Development 143, 2249–2260 (2016).
Labarrere, C. A., Dabiri, A. E. & Kassab, G. S. Thrombogenic and inflammatory reactions to biomaterials in medical devices. Front. Bioeng. Biotechnol. 8, 123 (2020).
Zheng, Y. et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl Acad. Sci. USA 109, 9342–9347 (2012).
Kelly, B. E. et al. Computed axial lithography (CAL): toward single step 3D printing of arbitrary geometries. Preprint at https://arxiv.org/abs/1705.05893 (2017).
Hutson, C. B. et al. Synthesis and characterization of tunable poly(ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng. A 17, 1713–1723 (2011).
Ye, W. et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 192, 108757 (2020).
Zhang, B. et al. Strengths, weaknesses, and applications of computational axial lithography in tissue engineering. Biodes. Manuf. 3, 5–6 (2020).
Hiob, M. A., She, S., Muiznieks, L. D. & Weiss, A. S. Biomaterials and modifications in the development of small-diameter vascular grafts. ACS Biomater. Sci. Eng. 3, 712–723 (2017).
Ravi, S. & Chaikof, E. L. Biomaterials for vascular tissue engineering. Regen. Med. 5, 107–120 (2010).
Jang, T. S. et al. 3D printing of hydrogel composite systems: recent advances in technology for tissue engineering. Int. J. Bioprint 4, 126 (2018).
Akentjew, T. L. et al. Rapid fabrication of reinforced and cell-laden vascular grafts structurally inspired by human coronary arteries. Nat. Commun. 10, 3098 (2019).
Pei, B. et al. Fiber-reinforced scaffolds in soft tissue engineering. Regen. Biomater. 4, 257–268 (2017).
Wang, Z. et al. 3D-printable self-healing and mechanically reinforced hydrogels with host–guest non-covalent interactions integrated into covalently linked networks. Mater. Horiz. 6, 733–742 (2019).
Zhang, J. et al. 3D printing of silk particle-reinforced chitosan hydrogel structures and their properties. ACS Biomater. Sci. Eng. 4, 3036–3046 (2018).
Cellular & gene therapy guidances, FDA https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances (2024).
LAVIV. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/laviv (2018).
Schurr, M. J. et al. Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J. Trauma 66, 866–874 (2009).
Grigoryan, B. et al. Development, characterization, and applications of multi-material stereolithography bioprinting. Sci. Rep. 11, 3171 (2021).
Camasão, D. B. & Mantovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Bio 10, 100106 (2021).
Erbel, R. & Eggebrecht, H. Aortic dimensions and the risk of dissection. Heart 92, 137–142 (2006).
Keelan, J. & Hague, J. P. The role of vascular complexity on optimal junction exponents. Sci. Rep. 11, 5408 (2021).
Sherman, T. F. On connecting large vessels to small. The meaning of Murray’s law. J. Gen. Physiol. 78, 431–453 (1981).
Painter, P. R., Edén, P. & Bengtsson, H. U. Pulsatile blood flow, shear force, energy dissipation and Murray’s law. Theor. Biol. Med. Model. 3, 31 (2006).
Morawietz, H. et al. Regulation of the endothelin system by shear stress in human endothelial cells. J. Physiol. 525, 761–770 (2000).
Taylor, D. J. et al. Refining our understanding of the flow through coronary artery branches; revisiting Murray’s law in human epicardial coronary arteries. Front. Physiol. 13, 871912 (2022).
Adam, J. A. Blood vessel branching: beyond the standard calculus problem. Math. Mag. 84, 196–207 (2011).
Xu, J. & Shi, G. P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta 1842, 2106–2119 (2014).
Tucker, W. D., Arora, Y. & Mahajan, K. Anatomy, blood vessels. in StatPearls [Internet] (StatPearls Publishing, 2023); https://pubmed.ncbi.nlm.nih.gov/29262226/
Russo, T. A., Banuth, A. M. M., Nader, H. B. & Dreyfuss, J. L. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS ONE 15, e0241040 (2020).
Kubota, Y., Kleinman, H. K., Martin, G. R. & Lawley, T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 107, 1589–1598 (1988).
Kalebic, T., Garbisa, S., Glaser, B. & Liotta, L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science 221, 281–283 (1983).
Marieb, E. N. Essentials of Human Anatomy & Physiology 11th edn (Pearson, 2015).
Courtney, J. M. & Sutherland, B. Harnessing the stem cell properties of pericytes to repair the brain. Neural Regen. Res. 15, 1021–1022 (2020).
Daneman, R. & Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015).
Hanrahan, V. et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma–carcinoma sequence during colorectal cancer progression. J. Pathol. 200, 183–194 (2003).
Holmes, D. I. R. & Zachary, I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 6, 209 (2005).
Chau, K., Hennessy, A. & Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 31, 782–786 (2017).
Baldwin, M. E. et al. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol. Cell. Biol. 25, 2441–2449 (2005).
Zhang, F. et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl Acad. Sci. USA 106, 6152–6157 (2009).
Achen, M. G. et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl Acad. Sci. USA 95, 548–553 (1998).
Duffy, A. M., Bouchier-Hayes, D. J. & Harmey, J. H. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF. in Madame Curie Bioscience Database [Internet] (Landes Bioscience, 2013); https://www.ncbi.nlm.nih.gov/books/NBK6482/
Bates, D. O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 87, 262–271 (2010).
Thurston, G. Complementary actions of VEGF and angiopoietin-1 on blood vessel growth and leakage. J. Anat. 200, 575–580 (2002).
Krilleke, D., Ng, Y.-S. E. & Shima, D. T. The heparin-binding domain confers diverse functions of VEGF-A in development and disease: a structure–function study. Biochem. Soc. Trans. 37, 1201–1206 (2009).
Park, J. E., Keller, G. A. & Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 4, 1317–1326 (1993).
Ruhrberg, C. et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16, 2684–2698 (2002).
Catena, R. et al. Increased expression of VEGF121/VEGF165–189 ratio results in a significant enhancement of human prostate tumor angiogenesis. Int. J. Cancer 120, 2096–2109 (2007).
Roskoski, R. VEGF receptor protein–tyrosine kinases: structure and regulation. Biochem. Biophys. Res. Commun. 375, 287–291 (2008).
Takahashi, T., Ueno, H. & Shibuya, M. VEGF activates protein kinase C-dependent, but Ras-independent Raf–MEK–MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18, 2221–2230 (1999).
Eliceiri, B. P. et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4, 915–924 (1999).
Chen, D. & Simons, M. Emerging roles of PLCγ1 in endothelial biology. Sci. Signal. 14, eabc6612 (2021).
Perrin, R. M. et al. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia 48, 2422–2427 (2005).
Acknowledgements
We thank J. S. Miller for guidance and helpful discussions. We acknowledge funding from the Defense Advanced Research Projects Agency under agreement numbers FA8650-21-1-7119 and AWD00001596, the Advanced Research Projects Agency for Health under award number AY1AX000003, and Breakthrough T1D awards 3-SRA-2025-1640-S-B, 3-SRA-2022-1255-S-B, 3-SRA-2023-1398-S-B, 3-SRA-2021-1023-S-B and 3-SRA-2024-1564-S-B.
Author information
Authors and Affiliations
Contributions
K.D.J. and S.P. contributed equally to researching, drafting and editing the majority of the paper and figures. J.W.R.S. researched and drafted numerous sections that discuss chemistry-based approaches for encouraging vascularization in vivo. J.D.W. provided valuable additions to the text and performed the final editing. J.D.H. and O.V. conceptualized the paper and supervised the writing.
Corresponding author
Ethics declarations
Competing interests
J.D.W. is a co-founder of and holds equity in ImmunoShield Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Shaochen Chen, Milica Radisic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janson, K.D., Parkhideh, S., Swain, J.W.R. et al. Strategies for the vascular patterning of engineered tissues for organ repair. Nat. Biomed. Eng 9, 1007–1025 (2025). https://doi.org/10.1038/s41551-025-01420-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01420-w