Abstract
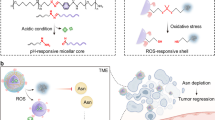
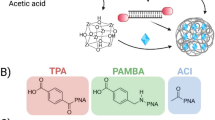
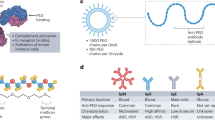
The high interfacial energy of nanomaterials limits their certain biomedical applications that require stealthiness to minimize non-specific interaction with biological components. While steric repulsion-based entropic stabilization—such as PEGylation—has long been the dominant strategy for designing stealth nanomaterials, its inherent softness and susceptibility to dynamic deformation and external forces often result in only moderate stealth performance. Here we report a distinct approach to achieving stealthiness by harnessing an ion-pair network, rather than maximizing steric repulsion. Using model polyion complex nanoparticles composed of equimolar charge ratios of polycations and polyanions, we demonstrate that increasing crosslinks between the constituent polyions beyond a critical threshold effectively reduces protein adsorption and macrophage uptake, enabling prolonged circulation with a half-life exceeding 100 hours. Building on this, we develop an asparaginase-loaded vesicular nanoreactor enveloped by a semi-permeable ion-pair network sheath for asparagine starvation therapy. The extended circulation of these nanoreactors enables sustained depletion of asparagine, leading to improved therapeutic outcomes for metastatic breast and pancreatic cancers. Our findings open an avenue for improving the pharmacokinetics of nanomaterials for therapeutic delivery through delicately engineering stable intermolecular structures with holistic cooperativity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files. All data generated in this study, including source data for the figures, are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Change history
26 November 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41551-025-01590-7
References
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Monopoli, M. P., Aberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012).
Yokoyama, M. et al. Polymer micelles as novel drug carrier: adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. J. Control. Release 11, 269–278 (1990).
Klibanov, A. L., Maruyama, K., Torchilin, V. P. & Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268, 235–237 (1990).
Kataoka, K., Kwon, G. S., Yokoyama, M., Okano, T. & Sakurai, Y. Block copolymer micelles as vehicles for drug delivery. J. Control. Release 24, 119–132 (1993).
Gref, R. et al. Biodegradable long-circulating polymeric nanospheres. Science 263, 1600–1603 (1994).
McPherson, T. B., Lee, S. J. & Park, K. Analysis of the prevention of protein adsorption by steric repulsion theory. ACS Symp. Ser. 602, 395–404 (1995).
Otsuka, H., Nagasaki, Y. & Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 55, 403–419 (2003).
Keefe, A. J. & Jiang, S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 4, 59–63 (2011).
Lu, Y. et al. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2, 318–325 (2018).
Nishiyama, N., Kato, Y., Sugiyama, Y. & Kataoka, K. Cisplatin-loaded polymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm. Res. 18, 1035–1041 (2001).
Nishiyama, N. et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 63, 8977–8983 (2003).
Cabral, H., Nishiyama, N., Okazaki, S., Koyama, H. & Kataoka, K. Preparation and biological properties of dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt)-loaded polymeric micelles. J. Control. Release 101, 223–232 (2005).
Kataoka, K., Harada, A. & Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 47, 113–131 (2001).
Fox, M. E., Szoka, F. C. & Frechet, J. M. J. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc. Chem. Res. 42, 1141–1151 (2009).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Perry, J. L. et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 12, 5304–5310 (2012).
Tockary, T. A. et al. Tethered PEG crowdedness determining shape and blood circulation profile of polyplex micelle gene carriers. Macromolecules 46, 6585–6592 (2013).
Yang, Q. et al. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol. Pharm. 11, 1250–1258 (2014).
Bertrand, N. et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 8, 777 (2017).
Cabral, H., Miyata, K., Osada, K. & Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 118, 6844–6892 (2018).
Cabral, H., Li, J., Miyata, K. & Kataoka, K. Controlling the biodistribution and clearance of nanomedicines. Nat. Rev. Bioeng. 2, 214–232 (2024).
Wen, P. et al. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 198, 114895 (2023).
Record, M. T., Anderson, C. F. & Lohman, T. M. Thermodynamic analysis of ion effects on binding and conformational equilibria of proteins and nucleic acids: roles of ion association or release, screening, and ion effects on water activity. Q. Rev. Biophys. 11, 103–178 (1978).
Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 25, 267–340 (1986).
Norde, W. Protein adsorption at solid surfaces: a thermodynamic approach. Pure Appl. Chem. 66, 491–496 (1994).
Hoffman, A. S. Non-fouling surface technologies. J. Biomater. Sci. Polym. Ed. 10, 1011–1014 (1999).
Vogler, E. A. Water and the acute biological response to surfaces. J. Biomater. Sci. Polym. Ed. 10, 1015–1045 (1999).
Achazi, K. et al. Understanding the interaction of polyelectrolyte architectures with proteins and biosystems. Angew. Chem. Int. Ed. 60, 3882–3904 (2021).
Olive, K. P. et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Koide, A. et al. Semipermeable polymer vesicle (PICsome) self-assembled in aqueous medium from a pair of oppositely charged block copolymers: physiologically stable micro-/nanocontainers of water-soluble macromolecules. J. Am. Chem. Soc. 128, 5988–5989 (2006).
Rocker, C., Potzl, M., Zhang, F., Parak, W. J. & Nienhaus, G. U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 4, 577–580 (2009).
Cedervall, T. et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl Acad. Sci. USA 104, 2050–2055 (2007).
Haroon, H. B., Hunter, A. C., Farhangrazi, Z. S. & Moghimi, S. M. A brief history of long circulating nanoparticles. Adv. Drug Deliv. Rev. 188, 114396 (2022).
Zhao, Z. M., Ukidve, A., Krishnan, V. & Mitragotri, S. Effect of physicochemical and surface properties on fate of drug nanocarriers. Adv. Drug Deliv. Rev. 143, 3–21 (2019).
Anselmo, A. C. et al. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 9, 3169–3177 (2015).
Kong, S. M., Costa, D. F., Jagielska, A., Van Vliet, K. J. & Hammond, P. T. Stiffness of targeted layer-by-layer nanoparticles impacts elimination half-life, tumor accumulation, and tumor penetration. Proc. Natl Acad. Sci. USA 118, e2104826118 (2021).
Kríz, J., Dautzenberg, H., Dybal, J. & Kurková, D. Competitive/cooperative electrostatic interactions in macromolecular complexes: multinuclear NMR study of PDADMAC-PMANa complexes in the presence of Al ions. Langmuir 18, 9594–9599 (2002).
Kríz, J. & Dautzenberg, H. Cooperative interactions of unlike macromolecules 2: NMR and theoretical study of electrostatic binding of sodium poly(styrenesulfonate)s to copolymers with variably distributed cationic groups. J. Phys. Chem. A 105, 3846–3854 (2001).
Mutaf, O. F., Anraku, Y., Kishimura, A. & Kataoka, K. Unilamellar polyion complex vesicles (PICsomes) with tunable permeabilities for macromolecular solutes with different shapes and sizes. Polymer 133, 1–7 (2017).
Klotz, I. M. Ligand-receptor complexes: origin and development of the concept. J. Biol. Chem. 279, 1–12 (2004).
Li, Q. S. et al. Zwitterionic biomaterials. Chem. Rev. 122, 17073–17154 (2022).
Nishida, K., Anada, T. & Tanaka, M. Roles of interfacial water states on advanced biomedical material design. Adv. Drug Deliv. Rev. 186, 114310 (2022).
Kataoka, K., Tsuruta, T., Akaike, T. & Sakurai, Y. Biomedical behavior of synthetic polyion complexes toward blood platelets. Makromol. Chem. 181, 1363–1373 (1980).
Flory, P. J. Molecular size distribution in three dimensional polymers. I. Gelation. J. Am. Chem. Soc. 63, 3083–3090 (1941).
Mittag, T. & Pappu, R. V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 82, 2201–2214 (2022).
Wada, A. & Nakamura, H. Nature of the charge distribution in proteins. Nature 293, 757–758 (1981).
Nakamura, H. Roles of electrostatic interaction in proteins. Q. Rev. Biophys. 29, 1–90 (1996).
Lai, S. K., Wang, Y. Y. & Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61, 158–171 (2009).
Ladd, J., Zhang, Z., Chen, S., Hower, J. C. & Jiang, S. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 9, 1357–1361 (2008).
Delgado, J. D. & Schlenoff, J. B. Static and dynamic solution behavior of a polyzwitterion using a Hofmeister salt series. Macromolecules 50, 4454–4464 (2017).
Li, S. D. et al. Tumor microenvironment-tailored weakly cell-interacted extracellular delivery platform enables precise antibody release and function. Adv. Funct. Mater. 29, 1903296 (2019).
Zhang, L. L. et al. Prolonging the plasma circulation of proteins by nano-encapsulation with phosphorylcholine-based polymer. Nano Res. 9, 2424–2432 (2016).
Huang, C. & Mason, J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc. Natl Acad. Sci. USA 75, 308–310 (1978).
Allen, C. et al. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol). Biosci. Rep. 22, 225–250 (2002).
Tirosh, O., Barenholz, Y., Katzhendler, J. & Priev, A. Hydration of polyethylene glycol-grafted liposomes. Biophys. J. 74, 1371–1379 (1998).
Garbuzenko, O., Barenholz, Y. & Priev, A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids 135, 117–129 (2005).
Arnold, K., Herrmann, A., Pratsch, L. & Gawrisch, K. The dielectric properties of aqueous solutions of poly(ethylene glycol) and their influence on membrane structure. Biochim. Biophys. Acta 815, 515–518 (1985).
Sorensen, K. K., Simon-Santamaria, J., McCuskey, R. S. & Smedsrod, B. Liver sinusoidal endothelial cells. Compr. Physiol. 5, 1751–1774 (2015).
Dirisala, A. et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 6, eabb8133 (2020).
Dawson, K. A. & Yan, Y. Current understanding of biological identity at the nanoscale and future prospects. Nat. Nanotechnol. 16, 229–242 (2021).
Jiang, K. et al. Kupffer cells determine intrahepatic traffic of PEGylated liposomal doxorubicin. Nat. Commun. 15, 6136 (2024).
Sueyoshi, D., Anraku, Y., Komatsu, T., Urano, Y. & Kataoka, K. Enzyme-loaded polyion complex vesicles as in vivo nanoreactors working sustainably under the blood circulation: characterization and functional evaluation. Biomacromolecules 18, 1189–1196 (2017).
Anraku, Y., Kishimura, A., Yamasaki, Y. & Kataoka, K. Living unimodal growth of polyion complex vesicles via two-dimensional supramolecular polymerization. J. Am. Chem. Soc. 135, 1423–1429 (2013).
Anraku, Y. et al. Systemically injectable enzyme-loaded polyion complex vesicles as in vivo nanoreactors functioning in tumors. Angew. Chem. Int. Ed. 55, 560–565 (2016).
Li, J., Anraku, Y. & Kataoka, K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew. Chem. Int. Ed. 59, 13526–13530 (2020).
Chen, H. et al. Polyion complex vesicles for photoinduced intracellular delivery of amphiphilic photosensitizer. J. Am. Chem. Soc. 136, 157–163 (2014).
Wen, P. et al. Engineering durable antioxidative nanoreactors as synthetic organelles for autoregulatory cellular protection against oxidative stress. J. Control. Release 382, 113683 (2025).
Dufour, E. et al. Pancreatic tumor sensitivity to plasma L-asparagine starvation. Pancreas 41, 940–948 (2012).
Chiu, M., Taurino, G., Bianchi, M. G., Kilberg, M. S. & Bussolati, O. Asparagine synthetase in cancer: beyond acute lymphoblastic leukemia. Front. Oncol. 9, 1480 (2020).
Lomelino, C. L., Andring, J. T., McKenna, R. & Kilberg, M. S. Asparagine synthetase: function, structure, and role in disease. J. Biol. Chem. 292, 19952–19958 (2017).
Keating, M. J., Holmes, R., Lerner, S. & Ho, D. H. L-Asparaginase and PEG asparaginase—past, present, and future. Leuk. Lymphoma 10, 153–157 (1993).
Xiao, Y. et al. Emerging therapies in cancer metabolism. Cell Metab. 35, 1283–1303 (2023).
Pieters, R. et al. L-Asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 117, 238–249 (2011).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
Li, J. & Kataoka, K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: the next generation. J. Am. Chem. Soc. 143, 538–559 (2021).
Gabriel, A. N. A. et al. Differences between KC and KPC pancreatic ductal adenocarcinoma mice models, in terms of their modeling biology and their clinical relevance. Pancreatology 20, 79–88 (2020).
Cabral, H. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815–823 (2011).
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Zhao, J. et al. Stromal modulation reverses primary resistance to immune checkpoint blockade in pancreatic cancer. ACS Nano 12, 9881–9893 (2018).
Hosein, A. N., Brekken, R. A. & Maitra, A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505 (2020).
Martin, J. D., Cabral, H., Stylianopoulos, T. & Jain, R. K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 17, 251–266 (2020).
Bear, A. S., Vonderheide, R. H. & O’Hara, M. H. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell 38, 788–802 (2020).
Löhr, M. et al. Transforming growth factor-β1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 61, 550–555 (2001).
Krall, A. S., Xu, S., Graeber, T. G., Braas, D. & Christofk, H. R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 7, 11457 (2016).
Rassolian, M., Chass, G. A., Setiadi, D. H. & Csizmadia, I. G. Asparagine—ab initio structural analyses. J. Mol. Struct. THEOCHEM 666-667, 273–278 (2003).
Kano, M. R. et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling. Proc. Natl Acad. Sci. USA 104, 3460–3465 (2007).
Kano, M. R. et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-β receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 100, 173–180 (2009).
Martin, J. D., Seano, G. & Jain, R. K. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol. 81, 505–534 (2019).
Martin, J. D. et al. Dexamethasone increases cisplatin-loaded nanocarrier delivery and efficacy in metastatic breast cancer by normalizing the tumor microenvironment. ACS Nano 13, 6396–6408 (2019).
Panagi, M. et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 10, 1910–1922 (2020).
Mpekris, F. et al. Normalizing the microenvironment overcomes vessel compression and resistance to nano-immunotherapy in breast cancer lung metastasis. Adv. Sci. 8, 2001917 (2021).
Qin, M. M., Feng, Z. H. & Meng, H. Enhanced transcytosis and retention (ETR) effect. Sci. Bull. 69, 3640–3643 (2024).
Tallal, L. et al. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer 25, 306–320 (1970).
Clarkson, B. et al. Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer 25, 279–305 (1970).
Hammel, P. et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized phase IIb trial. Eur. J. Cancer 124, 91–101 (2020).
Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2, 1–19 (2010).
Mononen, I. T., Kaartinen, V. M. & Williams, J. C. A fluorometric assay for glycosylasparaginase activity and detection of aspartylglycosaminuria. Anal. Biochem. 208, 372–374 (1993).
Szoka, F. & Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl Acad. Sci. USA 75, 4194–4198 (1978).
Acknowledgements
This research was supported financially by the Japan Science and Technology Agency (JST) through the Center of Innovation (COI) Program (JPMJCE1305 to K.K.) and the Open Innovation Platform for Industry-Academia Co-creation (COI-NEXT) Program (JPMJPF2202 to H.K. and K.K.), Grants-in-Aid for Early-Career Scientist (20K20209 to J.L.) and Scientific Research (B) (23H03740 to J.L.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT)/the Japan Society for the Promotion of Science (JSPS), and the JSPS International Research Fellowship (J.L., P17356; K.K. as the host researcher). This work was partially supported by Grants-in-Aid for Scientific Research (19H05720 and 22H00591 to M.T., 24K21109 to P.W.) from MEXT/JSPS and a grant for young researchers from the Hirose Foundation.
Author information
Authors and Affiliations
Contributions
J.L. and K.K. conceived of the ideas. J.L. designed and performed the experiments and analysed the data. J.L. prepared the figures and wrote the paper with input from all authors. P.W. helped with the preparation of PIC nanoparticles and in vitro investigation. K.T. and J.L. independently repeated intravital microscopic observation. J.F.R.V.G. helped with the NMR spectroscopic characterization. X.L. helped with the collection of blood and organs and the evaluation of antitumour efficacy. A.D., P.W., H.G. and H.C. contributed to the evaluation and discussion of biodistribution. Y.M. contributed to the inductively coupled plasma mass spectroscopy measurements. S.A. helped with the preparation of liposomes. H.K. helped with the flow cytometry measurements. Y.M. and Y.A. supplied some polymers. M.T. contributed to the discussion on the hydration effect. K.K. commented on the paper. J.L. and K.K. revised the paper. K.K. and J.L. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The patent application (PCT/JP2023/37954) related to this work has been filed (J.L. and K.K.). The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Martina Stenzel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Table 1 and legends for Videos 1–6.
Supplementary Video 1
Schematic illustration demonstrating the correlation between the stability of the ion-pair network sheath and nano–bio interactions.
Supplementary Video 2
IVRTCLSM imaging of blood vessels in the mouse ear dermis after injection of Cy5-labelled dePEGylated vesicles with 32.6% crosslinking (red).
Supplementary Video 3
IVRTCLSM imaging of the bloodstream FRET profile of Cy3/Cy5-co-labelled M39.5% nanoparticles in mouse ear dermis blood vessels. Stable FRET signal is demonstrated by a consistent Cy5/Cy3 ratio (yellow) in the imaging.
Supplementary Video 4
IVRTCLSM imaging of liver tissue after injection of Cy5-labelled dePEGylated vesicles with 32.6% crosslinking (red). The green fluorescence represents auto-fluorescence from liver parenchyma.
Supplementary Video 5
IVRTCLSM imaging of liver tissue after injection of Cy5-labelled M39.5% nanoparticles (red).
Supplementary Video 6
IVRTCLSM imaging of liver tissue after injection of Cy5-labelled V30.3% nanoparticles (red).
Supplementary Data
Source data for figures in the Supplementary Information.
Source data
Source Data Figs. 1–8
Statistical source data for Figs. 1–8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Toh, K., Wen, P. et al. Steric stabilization-independent stealth cloak enables nanoreactors-mediated starvation therapy against refractory cancer. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01534-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41551-025-01534-1