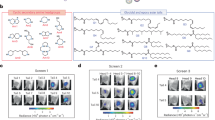
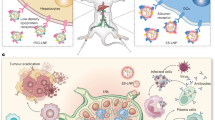
Abstract
Two key challenges in translating messenger RNA-based lipid nanoparticle (mRNA-LNP) cancer vaccines to clinical use are limited mRNA expression and unavoidable inflammatory responses. Here we develop a membrane-destabilizing zwitterionic ionizable lipid that enhances mRNA expression by promoting endosomal escape while reducing inflammatory reactogenicity. This lipid features a pyridine-based carboxybetaine (PyCB) zwitterionic headgroup, biodegradable multitailed alkyl chains and a tertiary amine linker. The PyCB headgroup forms a zwitterionic PyCB–water complex that protonates to a positively charged state below pH 6.8. This allows for good biocompatibility at physiological pH and strong protonation in endosomes, enabling earlier and more efficient mRNA release when synergistic with the tertiary amine and the tail moiety. Incorporating membrane-destabilizing zwitterionic lipids into the LNP formulation used in a commercially available mRNA vaccine significantly boosts mRNA expression in antigen-presenting cells within lymph nodes, enhancing cytotoxic T cell activation. In addition, these membrane-destabilizing zwitterionic lipid-containing nanoparticles show reduced inflammation and neutrophil infiltration at the injection site due to their zwitterionic property. These lipids are also compatible with existing targeted nanoparticle formulations, further improving mRNA delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its supplementary files. Any additional requests for information can be directed to and will be fulfilled by the corresponding author. Source data are provided with this paper.
References
Liu, H. et al. Beyond the endosomal bottleneck: understanding the efficiency of mRNA/LNP delivery. Adv. Funct. Mater. 34, 2404510 (2024).
Sellars, M. C., Wu, C. J. & Fritsch, E. F. Cancer vaccines: building a bridge over troubled waters. Cell 185, 2770–2788 (2022).
Munro, A. P. et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 22, 1131–1141 (2022).
Pulendran, B., Arunachalam, S. P. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Rosa Duque, J. S. et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat. Commun. 13, 3700 (2022).
Korzun, T. et al. From bench to bedside: implications of lipid nanoparticle carrier reactogenicity for advancing nucleic acid therapeutics. Pharmaceuticals 16, 1088 (2023).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Li, B. et al. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat. Biomed. Eng. 9, 167–184 (2025).
Wittrup, A. et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33, 870–876 (2015).
Bauernfeind, S. et al. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines 9, 1089 (2021).
Zhao, Y., Wang, Z. M., Song, D., Chen, M. & Xu, Q. Rational design of lipid nanoparticles: overcoming physiological barriers for selective intracellular mRNA delivery. Curr. Opin. Chem. Biol. 81, 102499 (2024).
Albertsen, C. H. et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 188, 114416 (2022).
Chatterjee, S., Kon, E., Sharma, P. & Peer, D. Endosomal escape: a bottleneck for LNP-mediated therapeutics. Proc. Natl Acad. Sci. USA 121, e2307800120 (2024).
Witten, J., Hu, Y., Langer, R. & Anderson, D. G. Recent advances in nanoparticulate RNA delivery systems. Proc. Natl Acad. Sci. USA 121, e2307798120 (2024).
Jiang, S. & Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 22, 920–932 (2010).
Bai, T. et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 25, 1566–1575 (2019).
Luozhong, S. et al. A de novo strategy to improve pharmacokinetics of proteins from mRNA therapeutics via zwitterionic polypeptide fusion. J. Am. Chem. Soc. 146, 21245–21249 (2024).
Yuan, Z. et al. Mitigating the immunogenicity of AAV-mediated gene therapy with an immunosuppressive phosphoserine-containing zwitterionic peptide. J. Am. Chem. Soc. 144, 20507–20513 (2022).
Dong, D. et al. High-strength and fibrous capsule-resistant zwitterionic elastomers. Sci. Adv. 7, eabc5442 (2021).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Zhou, K. et al. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. Int. Ed. Engl. 50, 6109–6114 (2011).
Sayers, E. J. et al. Endocytic profiling of cancer cell models reveals critical factors influencing LNP-mediated mRNA delivery and protein expression. Mol. Ther. 27, 1950–1962 (2019).
Zhao, Y., Li, Q., Chai, J. & Liu, Y. Cargo-templated crosslinked polymer nanocapsules and their biomedical applications. Adv. NanoBiomed Res. 1, 2000078 (2021).
Pattipeiluhu, R. et al. Liquid crystalline inverted lipid phases encapsulating siRNA enhance lipid nanoparticle mediated transfection. Nat. Commun. 15, 1303 (2024).
Yu, H. et al. Inverse cubic and hexagonal mesophase evolution within ionizable lipid nanoparticles correlates with mRNA transfection in macrophages. J. Am. Chem. Soc. 145, 24765–24774 (2023).
Han, X. et al. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 12, 7233 (2021).
Philipp, J. et al. pH-dependent structural transitions in cationic ionizable lipid mesophases are critical for lipid nanoparticle function. Proc. Natl Acad. Sci. USA 120, e2310491120 (2023).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Wang, D. Y. et al. Liposomes with water as a pH-responsive functionality for targeting of acidic tumor and infection sites. Angew. Chem. Int. Ed. Engl. 60, 17714–17719 (2021).
Sagarik, K. & Chaiyapongs, S. Structures and stability of salt-bridge in aqueous solution. Biophys. Chem. 117, 119–140 (2005).
Gun’ko, V. M. & Turov, V. V. Structure of hydrogen bonds and 1H NMR spectra of water at the interface of oxides. Langmuir 15, 6405–6415 (1999).
Ou, H. et al. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 65, 339–348 (2018).
Zhang, P. et al. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl Acad. Sci. USA 112, 12046–12051 (2015).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Förster, R., Braun, A. & Worbs, T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 33, 271–280 (2012).
Zhao, Y. et al. Antitumour vaccination via the targeted proteolysis of antigens isolated from tumour lysates. Nat. Biomed. Eng 9, 234–248 (2025).
Yamashiro, D. J. & Maxfield, F. R. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J. Cell Biol. 105, 2723–2733 (1987).
Schlame, M. et al. The physical state of lipid substrates provides transacylation specificity for tafazzin. Nat. Chem. Biol. 8, 862–869 (2012).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
Zhao, Y. et al. Nanomechanical action opens endo-lysosomal compartments. Nat. Commun. 14, 6645 (2023).
Wang, C., Zhao, C., Wang, W., Liu, X. & Deng, H. Biomimetic noncationic lipid nanoparticles for mRNA delivery. Proc. Natl Acad. Sci. USA 120, e2311276120 (2023).
Phillipson, M. & Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390 (2011).
Nauseef, W. M. & Borregaard, N. Neutrophils at work. Nat. Immunol. 15, 602–611 (2014).
Vaure, C. & Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 5, 316 (2014).
Chaudhary, N. et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng. 8, 1483–1498 (2024).
Capece, D. et al. NF-kappaB: blending metabolism, immunity, and inflammation. Trends Immunol. 43, 757–775 (2022).
Li, A. W. et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 17, 528–534 (2018).
Acknowledgements
We acknowledge financial support from the National Institute of Allergy and Infectious Disease (1R01AI178125-01A1 and 1R01AI193334-01) and start-up support from Cornell University, including Robert Langer ’70 Family and Friends Professorship, Cornell NEXT Nano Initiative and Cornell Engineering’s inaugural Sprout Award. Animal IVIS images were acquired through the Cornell Institute of Biotechnology’s Imaging Facility with NIH S10OD025049. We thank the Weiss lab at Cornell for the kind gift of EO771 cells; T. Totman, E. Feldman and F. Burgus at Cornell CARE for service and advice on caring for animals; the Cornell IACUC for help with composing and managing animal protocols; and the NIH Tetramer Core Facility (NIH Contract 75N93020D00005 and RRID:SCR_026557) for providing anti-H2Kb OVA tetramer-SIINFEKL antibody. Elements in Supplementary Fig. 7 were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Y.Z. and S.J. devised the project and wrote the paper. Y.Z., R.L., P.L., J.W., Y.C., Y.M., Z.C., M.C. and S.L. carried out the experimental work and analysed the data. E.W. and A.L. provided the supplies for cell experiments. Y.D., Y.H., Z.T., C.T., S.C., H.Y., D.L., W.G. and S.B. contributed to the discussion of the experimental results.
Corresponding author
Ethics declarations
Competing interests
S.J. and Y.Z. are authors of a US Provisional Application (No. 63/723,698) related to this work filed on 22 November 2024 and S.J., Y.Z., Y.H. and P.L. an International (PCT) Application (No. PCT/US2025/056506) filed on 21 November 2025 by Cornell University. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14.
Supplementary Data 1
Source data for supplementary figures.
Source data
Source Data Figs. 1–6
Source data for Figs. 1–6.
Source Data Fig. 4
Source data Figs. 4l–n.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Li, R., Liu, P. et al. Low reactogenicity and high tumour antigen expression from mRNA-LNPs with membrane-destabilizing zwitterionic lipids. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01577-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41551-025-01577-4