Abstract
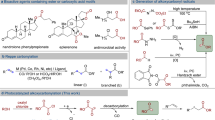
Alkenes serve as versatile building blocks in diverse organic transformations. Despite notable advancements in olefination methods, a general strategy for the direct conversion of carboxylic acids, alcohols and alkanes into alkenes remains a formidable challenge owing to their inherent reactivity disparities. Here we demonstrate an integrated photochemical strategy that facilitates a one-pot conversion of these fundamental building blocks into alkenes through a sequential C(sp3)–C(sp3) bond formation–fragmentation process, utilizing an easily accessible and recyclable phenyl vinyl ketone as the ‘olefination reagent’. This practical method not only offers an unparalleled paradigm for accessing value-added alkenes from abundant and inexpensive starting materials but also showcases its versatility through various complex scenarios, including late-stage on-demand olefination of multifunctional molecules, chain homologation of acids and concise syntheses of bioactive molecules. Moreover, initiating from carboxylic acids, alcohols and alkanes, this protocol presents a complementary approach to traditional olefination methods, making it a highly valuable addition to the research toolkit for alkene synthesis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that all the data supporting the findings of this study are available within the article and its Supplementary Information.
References
Patai, S. The Chemistry of Alkenes (Wiley, 1964).
Clayden, J., Greeves, N., Warren, S. & Wothers, P. Organic Chemistry (Oxford Univ. Press, 2011).
Takeda, T. Modern carbonyl olefination—methods and applications. Synthesis 2004, 1532 (2004).
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007).
Ertl, P. & Schuhmann, T. A systematic cheminformatics analysis of functional groups occurring in natural products. J. Nat. Prod. 82, 1258–1263 (2019).
Zhang, X., Jordan, F. & Szostak, M. Transition-metal-catalyzed decarbonylation of carboxylic acids to olefins: exploiting acyl C–O activation for the production of high value products. Org. Chem. Front. 5, 2515–2521 (2018).
Chatterjee, A. & Jensen, V. R. A heterogeneous catalyst for the transformation of fatty acids to α-olefins. ACS Catal. 7, 2543–2547 (2017).
Sun, X., Chen, J. & Ritter, T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nat. Chem. 10, 1229–1233 (2018).
Nguyen, V. T. et al. Alkene synthesis by photocatalytic chemoenzymatically compatible dehydrodecarboxylation of carboxylic acids and biomass. ACS Catal. 9, 9485–9498 (2019).
Tlahuext-Aca, A., Candish, L., Garza-Sanchez, R. A. & Glorius, F. Decarboxylative olefination of activated aliphatic acids enabled by dual organophotoredox/copper catalysis. ACS Catal. 8, 1715–1719 (2018).
Garrido-Castro, A. et al. Scalable electrochemical decarboxylative olefination driven by alternating polarity. Angew. Chem. Int. Ed. 62, e202309157 (2023).
Lebel, H. & Paquet, V. Multicatalytic processes using diverse transition metals for the synthesis of alkenes. J. Am. Chem. Soc. 126, 11152–11153 (2004).
Merza, F., Taha, A. & Thiemann, T. Tandem-, domino- and one-pot reactions involving Wittig- and Horner-Wadsworth-Emmons olefination. Alkenes https://doi.org/10.5772/intechopen.70364 (InTech, 2017).
Chakraborty, S., Das, U. K., Ben-David, Y. & Milstein, D. Manganese catalyzed α-olefination of nitriles by primary alcohols. J. Am. Chem. Soc. 139, 11710–11713 (2017).
Gordon, B. M., Lease, N., Emge, T. J., Hasanayn, F. & Goldman, A. S. Reactivity of iridium complexes of a triphosphorus-pincer ligand based on a secondary phosphine. Catalytic alkane dehydrogenation and the origin of extremely high activity. J. Am. Chem. Soc. 144, 4133–4146 (2022).
Jia, X. & Huang, Z. Conversion of alkanes to linear alkylsilanes using an iridium–iron-catalysed tandem dehydrogenation–isomerization–hydrosilylation. Nat. Chem. 8, 157–161 (2016).
Wang, K. et al. Selective dehydrogenation of small and large molecules by a chloroiridium catalyst. Sci. Adv. 8, eabo6586 (2022).
Voica, A., Mendoza, A., Gutekunst, W. R., Fraga, J. O. & Baran, P. S. Guided desaturation of unactivated aliphatics. Nat. Chem. 4, 629–635 (2012).
Parasram, M., Chuentragool, P., Wang, Y., Shi, Y. & Gevorgyan, V. General, auxiliary-enabled photoinduced Pd-catalyzed remote desaturation of aliphatic alcohols. J. Am. Chem. Soc. 139, 14857–14860 (2017).
West, J., Huang, D. & Sorensen, E. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun. 6, 10093 (2015).
Zhou, M.-J., Zhang, L., Liu, G., Xu, C. & Huang, Z. Site-selective acceptorless dehydrogenation of aliphatics enabled by organophotoredox/cobalt dual catalysis. J. Am. Chem. Soc. 143, 16470–16485 (2021).
Kumar, A., Bhatti, T. M. & Goldman, A. S. Dehydrogenation of alkanes and aliphatic groups by pincer-ligated metal complexes. Chem. Rev. 117, 12357–12385 (2017).
Edwards, J. T. et al. Decarboxylative alkenylation. Nature 545, 213–218 (2017).
Coyle, J. D. & Carless, H. A. J. Selected aspects of photochemistry. I Photochemistry of carbonyl compounds. Chem. Soc. Rev. 1, 465–480 (1972).
Majhi, S. Applications of Norrish type I and II reactions in the total synthesis of natural products: a review. Photochem. Photobiol. Sci. 20, 1357–1378 (2021).
Ito, Y. Photochemistry for Biomedical Applications (Springer Nature, 2018).
Ledford, B. E. & Carreira, E. M. Total synthesis of (+)-trehazolin: optically active spirocycloheptadienes as useful precursors for the synthesis of aminocyclopentitols. J. Am. Chem. Soc. 117, 11811–11812 (1995).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2010).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 1, 523–598 (2021).
Tang, H. et al. Direct synthesis of thioesters from feedstock chemicals and elemental sulfur. J. Am. Chem. Soc. 145, 5846–5854 (2023).
Cao, H. et al. Photo-induced decarboxylative Heck-type coupling of unactivated aliphatic acids and terminal alkenes in the absence of sacrificial hydrogen acceptors. J. Am. Chem. Soc. 140, 16360–16367 (2018).
Beil, S. B., Chen, T. Q., Intermaggio, N. E. & MacMillan, D. W. C. Carboxylic acids as adaptive functional groups in metallaphotoredox catalysis. Acc. Chem. Res. 55, 3481–3494 (2022).
Anwar, K., Merkens, K., Aguilar Troyano, F. J. & Gómez‐Suárez, A. Radical deoxyfunctionalisation strategies. Eur. J. Org. Chem. https://doi.org/10.1002/ejoc.202200330 (2022).
Dong, Z. & MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Wang, J. Z., Sakai, H. A. & MacMillan, D. W. C. Alcohols as alkylating agents: photoredox-catalyzed conjugate alkylation via in situ deoxygenation. Angew. Chem. Int. Ed. 61, e202207150 (2022).
Cao, H. et al. Brønsted acid-enhanced direct hydrogen atom transfer photocatalysis for selective functionalization of unactivated C(sp3)–H bonds. Nat. Synth. 1, 794–803 (2022).
Wagner, P. J. et al. Type II photoprocesses of phenyl ketones. Glimpse at the behavior of 1,4 biradicals. J. Am. Chem. Soc. 94, 7506–7512 (1972).
Oelgemöller, M. & Hoffmann, N. Studies in organic and physical photochemistry—an interdisciplinary approach. Org. Biomol. Chem. 14, 7392–7442 (2016).
Christianson, D. W., Baggio, R. & Elbaum, D. Compositions and methods for inhibiting arginase activity. US patent 6,387,890B1 (2002).
Lappin, G. R. Alpha Olefins Applications Handbook (CRC Press, 2019).
Monti, L., Berliner, D. L., Jennings-White, C. L. & Adams, N. W. 17-Methylene-androstan-3α-ol analogs as CRH inhibitors. International patent WO 2002089814A1 (2002).
Ziegler, F. E., Berlin, M. Y., Lee, K. & Looker, A. R. Formation of 9,10-unsaturation in the mitomycins: facile fragmentation of β-alkyl-β-aryl-α-oxo-γ-butyrolactones. Org. Lett. 2, 3619–3621 (2000).
Schwertz, G. et al. Synthesis of amorpha-4,11-diene from dihydroartemisinic acid. Tetrahedron 75, 743–748 (2019).
Tückmantel, W. & Kozikowski, A. P. Intermediates useful for the synthesis of huperzine A. US patent 6,271,379B1 (2001).
Coughlin, D. J. & Salomon, R. G. New synthetic approach to 4-alkyIidenecyclohexenes. Reduction-protodesilylation of benzylsilanes. J. Org. Chem. 22, 3784–3790 (1979).
Pollini, G. P., Benetti, S., Risi, C. D. & Zanirato, V. Hagemann’s ester: a timeless building block for natural product synthesis. Tetrahedron 66, 2775–2802 (2010).
Levterov, V. V. et al. 2-Oxabicyclo[2,2,2]octane as a new bioisostere of the phenyl ring. Nat. Commun. 14, 5608 (2023).
Bugarin, A., Jones, K. D. & Connell, B. T. Efficient, direct α-methylenation of carbonyls mediated by diisopropylammonium trifluoroacetate. Chem. Commun. 46, 1715–1717 (2010).
Song, L. et al. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 5, 832–838 (2022).
Börjesson, M., Moragas, T. & Martin, R. Ni-catalyzed carboxylation of unactivated alkyl chlorides with CO2. J. Am. Chem. Soc. 138, 7504–7507 (2016).
Parikh, A., Parikh, H. & Parikh, K. Arndt-Eistert Reaction. Name Reactions in Organic Synthesis (ed Surrey, A. R.) 3–6 (Foundation Books, 2006).
Rodriguez, C. R. et al. Synthesis and biological activity of fluorinated analogues of the DAF-12 receptor antagonist 24-hydroxy-4-cholen-3-one. Steroids 151, 108469 (2019).
Zhang, Z.-Q. et al. Difluoromethylation of unactivated alkenes using freon-22 through tertiary amine-borane-triggered halogen atom transfer. J. Am. Chem. Soc. 144, 14288–14296 (2022).
Stefani, H. A., Costa, I. M. & Zeni, G. Synthesis of polyacetylenic montiporic acids A and B. Tetrahedron Lett. 40, 9215–9217 (1999).
Acknowledgements
We thank the National Research Foundation, the Prime Minister’s Office of Singapore under its NRF-CRP programme (award NRFCRP25-2020RS-0002), the Ministry of Education (MOE) of Singapore (MOET2EP10120-0014 and MOET2EP10121-0004), NUS (Suzhou) Research Institute, National Natural Science Foundation of China (grant nos. 22071170 and 92156025) and the National Key Research and Development Program of China (2019YFA0905100) for the financial support provided. We thank H. Ting Ang (NUS), M. Wai Liaw (NUS) and H. Cao (University of Basel) for their valuable proofreading assistance on the manuscript.
Author information
Authors and Affiliations
Contributions
H.Z., J.-A.M., Y.Z. and J.W. designed and analysed the experiments. H.Z. and R.Y. conducted the experiments. H.Z., J.-A.M., Y.Z. and J.W. wrote the manuscript. J.W. guided the whole project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Zhiwei Zuo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22, Tables 1–13, Materials and methods, Experimental procedures, Mechanistic studies, Characterization data, Substrate limitation and extension and NMR spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, H., Yin, R., Zhao, Y. et al. Modular alkene synthesis from carboxylic acids, alcohols and alkanes via integrated photocatalysis. Nat. Chem. 16, 1822–1830 (2024). https://doi.org/10.1038/s41557-024-01642-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01642-6
This article is cited by
-
Enantioconvergent deoxygenative reductive cross-coupling of lactic acid derivatives
Science China Chemistry (2025)