Abstract
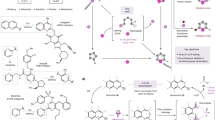
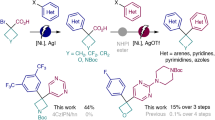
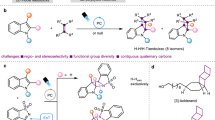
The rapid diversification of core ring structures in complex molecules through switchable skeletal editing is valuable in the drug discovery process. However, controllable methods for chemically divergent modifications of azaarene frameworks using common substrates are challenging, despite the potential to maximize structural diversity and complexity. Here we report the tunable skeletal editing of quinolines through Brønsted acid-catalysed multicomponent reactions of quinoline N-oxides, dialkyl acetylenedicarboxylates and water to generate nitrogen-containing heteroaromatic compounds together with linear compounds in a modular fashion. Specifically, in a one-pot procedure, after cyclization and sequential rearrangement processes, the quinoline N-oxides are easily converted into unique 2-substituted indolines. These then undergo acid-promoted fragmentation to give indoles, base-facilitated ring-opening to afford 2-alkenylanilines and oxidative cyclization to yield isoquinolinones. Catalytic asymmetric skeletal editing of quinolines is also realized, providing enantioenriched benzazepines bearing quaternary stereocentres, and late-stage skeletal modification of quinoline cores in several drugs is demonstrated.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2334869 (84), CCDC 2401914 (103), CCDC 2385984 (109) and CCDC 2334870 (110). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Woo, J. et al. Scaffold hopping by net photochemical carbon deletion of azaarenes. Science 376, 527–532 (2022).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Callis, T. B., Garrett, T. R., Montgomery, A. P., Danon, J. J. & Kassiou, M. Recent scaffold hopping applications in central nervous system drug discovery. J. Med. Chem. 65, 13483–13504 (2022).
Hu, Y., Stumpfe, D. & Bajorath, J. Recent advances in scaffold hopping. J. Med. Chem. 60, 1238–1246 (2017).
Joynson, B. W. & Ball, L. T. Skeletal editing: interconversion of arenes and heteroarenes. Helv. Chim. Acta 106, e202200182 (2023).
Wang, H. et al. Dearomative ring expansion of thiophenes by bicyclobutane insertion. Science 381, 75–81 (2023).
Díaz-Requejo, M. M. & Pérez, P. J. Coinage metal catalyzed C-H bond functionalization of hydrocarbons. Chem. Rev. 108, 3379–3394 (2008).
Fan, Z. et al. Molecular editing of aza-arene C–H bonds by distance, geometry and chirality. Nature 610, 87–93 (2022).
Suzuki, T., Hamura, T. & Suzuki, K. Ring selectivity: successive ring expansion of two benzocyclobutenes for divergent access to angular and linear benzanthraquinones. Angew. Chem. Int. Ed. 47, 2248–2252 (2008).
Bi, H. & Wang, S. R. Modular regiodivergent synthesis of benzo-fused isocoumarins by a cyclopropane aromatization strategy. Org. Lett. 24, 6316–6320 (2022).
Schmitt, H. L. et al. Regiodivergent ring-expansion of oxindoles to quinolinones. J. Am. Chem. Soc. 146, 4301–4308 (2024).
Liu, S. et al. Tunable molecular editing of indoles with fluoroalkyl carbenes. Nat. Chem. 16, 988–997 (2024).
Uhlenbruck, B. J. H., Josephitis, C. M., Lescure, L., Paton, R. S. & McNally, A. A deconstruction–reconstruction strategy for pyrimidine diversification. Nature 631, 87–93 (2024).
Woo, J., Stein, C., Christian, A. H. & Levin, M. D. Carbon-to-nitrogen single-atom transmutation of azaarenes. Nature 623, 77–82 (2023).
Baumann, M. & Baxendale, I. R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 9, 2265–2319 (2013).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Kallitsis, J. K., Geormezi, M. & Neophytides, S. G. Polymer electrolyte membranes for high-temperature fuel cells based on aromatic polyethers bearing pyridine units. Polym. Int. 58, 1226–1233 (2009).
Zhou, F. & Jiao, L. Recent developments in transition-metal-free functionalization and derivatization reactions of pyridines. Synlett 32, 159–178 (2021).
Boudry, E., Bourdreux, F., Marrot, J., Moreau, X. & Ghiazza, C. Dearomatization of pyridines: photochemical skeletal enlargement for the synthesis of 1,2-diazepines. J. Am. Chem. Soc. 146, 2845–2854 (2024).
Cheng, Q. et al. Skeletal editing of pyridines through atom-pair swap from CN to CC. Nat. Chem. 16, 741–748 (2024).
Roswell, B. R., Zhao, Z., Gonciarz, R. L. & Pandya, K. M. Regioselective pyridine to benzene edit inspired by water-displacement. J. Am. Chem. Soc. 146, 19660–19666 (2024).
Li, L., Chen, Z., Zhang, X. & Jia, Y. Divergent strategy in natural product total synthesis. Chem. Rev. 118, 3752–3832 (2018).
Musonda, C. C. et al. Application of multi-component reactions to antimalarial drug discovery. Part 1: Parallel synthesis and antiplasmodial activity of new 4-aminoquinoline Ugi adducts. Bioorg. Med. Chem. Lett. 14, 3901–3905 (2004).
Dömling, A., Achatz, S. & Beck, B. Novel anti-tuberculosis agents from MCR libraries. Bioorg. Med. Chem. Lett. 17, 5483–5486 (2007).
Ishiguro, Y., Funakoshi, K., Saeki, S. & Hamana, M. The reaction of 2-substituted quinoline 1-oxides with dimethyl acetylenedicarboxylate: formation of 1-benzazepine derivatives. Heterocycles 20, 1545–1547 (1983).
Ryzhakov, A. V. & Rodina, L. L. Aromatic N-oxides as 1,3-dipoles and π-donors in reactions with unsaturated compounds. Review. Chem. Heterocycl. Compd 28, 483–493 (1992).
Ochiai, E. Recent Japanese work on the chemistry of pyridine 1-oxide and related compounds. J. Org. Chem. 18, 534–551 (1953).
Bull, J. A., Mousseau, J. J., Pelletier, G. & Charette, A. B. Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N-activated pyridines. Chem. Rev. 112, 2642–2713 (2012).
Liu, X. & Qin, Y. Indole alkaloid synthesis facilitated by photoredox catalytic radical cascade reactions. Acc. Chem. Res. 52, 1877–1891 (2019).
Zhang, Y., Jiang, F. & Shi, F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: strategies, reactions, and outreach. Acc. Chem. Res. 53, 425–446 (2020).
Tomioka, Y., Nagahiro, C., Nomura, Y. & Maruoka, H. Synthesis and 1,3-dipolar cycloaddition reactions of N-aryl-C,C-dimethoxycarbonylnitrones. J. Heterocyclic. Chem. 40, 121–127 (2003).
Yang, H., Wei, Y. & Shi, M. Construction of spiro[indoline]oxindoles through one-pot thermal-induced [3+2] cycloaddition/silica gel-promoted fragmentation sequence between isatin ketonitrones and electron-deficient alkynes. Tetrahedron 69, 4088–4097 (2013).
Jiang, H., Gao, H., Liu, B. & Wu, W. Palladium-catalyzed selective aminoamidation and aminocyanation of alkenes using isonitrile as amide and cyanide sources. Chem. Commun. 50, 15348–15351 (2014).
Baciocchi, E., Dell'Aira, D. & Ruzziconi, R. Dimethyl arylmalonates from cerium(IV) ammonium nitrate promoted reactions of dimethylmalonate with aromatic compounds in methanol. Tetrahedron Lett. 27, 2763–2766 (1986).
Zhang, Y. & Li, C. DDQ-mediated direct cross-dehydrogenative-coupling (CDC) between benzyl ethers and simple ketones. J. Am. Chem. Soc. 128, 4242–4243 (2006).
Martino, E. et al. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 27, 701–707 (2017).
Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 148, 104398 (2019).
Fleming, G. S. & Beeler, A. B. Regioselective and enantioselective intermolecular Buchner ring expansions in flow. Org. Lett. 19, 5268–5271 (2017).
Zhang, X. et al. Asymmetric dearomative single-atom skeletal editing of indoles and pyrroles. Nat. Chem. 17, 215–225 (2025).
Kricka, L. J. & Ledwith, A. Dibenz[b,f]azepines and related ring systems. Chem. Rev. 74, 101–123 (1974).
Pindur, U. & Flo, C. First reactions of dialkoxycarbenium tetrafluoroborates with pyrroles, 5H-dibenz[b,f]azepines, and electron-rich arenes. J. Heterocyclic. Chem. 26, 1563–1568 (1989).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Maier, G. The norcaradiene problem. Angew. Chem. Int. Ed. 6, 402–413 (1967).
Bi, H., Shen, C. & Wang, S. R. Catalytic dearomative [1,5]-sigmatropic carbon shift of heterole-fused norcaradienes enabled concise helicenation. Angew. Chem. Int. Ed. 64, e202415839 (2024).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant nos. 22371183 and 22101172 to H.W.). We thank L.-L. Li for the X-ray structural analysis of compounds 84, 103, 109 and 110.
Author information
Authors and Affiliations
Contributions
D.T., Y.-P.H., L.-S.Y. and Z.-C.L. designed the experiments and collected and analysed the data. D.T. optimized the reaction conditions and performed the experiments. Y.-P.H. determined and optimized the initial reaction conditions for asymmetric skeletal editing. L.-S.Y. and Z.-C.L. contributed to the preparation of substrates. H.W. supervised the research and conceived the project. H.W. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Sunewang R. Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1, Tables 1–11, experimental procedures, synthetic procedures, characterization data, NMR spectra and HPLC traces.
Supplementary Data 1
Crystallographic data for compound 84; CCDC reference 2334869.
Supplementary Data 2
Structure factors of compound 84; CCDC reference 2334869.
Supplementary Data 3
Crystallographic data for compound 103; CCDC reference 2401914.
Supplementary Data 4
Structure factors of compound 103; CCDC reference 2401914.
Supplementary Data 5
Crystallographic data for compound 109; CCDC reference2385984.
Supplementary Data 6
Structure factors of compound 109; CCDC reference 2385984.
Supplementary Data 7
Crystallographic data for compound 110; CCDC reference 2334870.
Supplementary Data 8
Structure factors of compound 110; CCDC reference 2334870.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, D., He, YP., Yang, LS. et al. Switchable skeletal editing of quinolines enabled by cyclizative sequential rearrangements. Nat. Chem. 17, 952–960 (2025). https://doi.org/10.1038/s41557-025-01793-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01793-0
This article is cited by
-
Skeletal editing of 4-arylpyrimidines into diverse nitrogen heteroaromatics via four-atom synthons
Nature Communications (2025)