Abstract
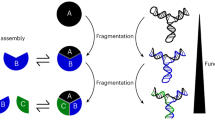
Selective formation of multicomponent structures via the self-assembly of numerous building blocks is ubiquitous in biological systems but challenging to emulate synthetically. More components introduce additional possibilities for kinetic intermediates with trap-state ability, hampering access to desired products. In covalent chemistry, templates, reagents and catalysts are applied to create alternative pathways for desired product formation. Analogously, we enlist exo-templating to mould the formation of large, multicomponent supramolecular structures. Specifically, a charged ring docks at 1,5-dioxynaphthalene stations within exo-functionalized building blocks to promote formation of cuboctahedral Pd12L24 nanospheres via exoskeletal templating. With the exo-templating ring present, nanosphere formation occurs via small Pdx–Ly oligomers, while in the absence of the ring a Pdx–Ly polymer resting state rapidly evolves, from which nanosphere formation occurs slowly. We demonstrate a form of kinetic templating—via intermediate destabilization—resembling properties observed in catalysis. Importantly, unlike typically employed endo-templates, we demonstrate that exo-templating is particularly suited for larger, complex, self-assembled structures.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw data generated during this study are available in the Supplementary Information. All raw data that support the findings of this study are available via Figshare at https://doi.org/10.6084/m9.figshare.28163420.v1 (ref. 85). Source data are provided with this paper.
Code availability
Input and automation codes for AMBER simulations are supplied with computational results and are available via Figshare at https://doi.org/10.6084/m9.figshare.28163420.v1 (ref. 85).
References
Rizzuto, F. J., von Krbek, L. K. S. & Nitschke, J. R. Strategies for binding multiple guests in metal–organic cages. Nat. Rev. Chem. 3, 204–222 (2019).
Gramage-Doria, R. et al. Gold(I) catalysis at extreme concentrations inside self-assembled nanospheres. Angew. Chem. Int. Ed. Engl. 53, 13380–13384 (2014).
Jongkind, L. J., Elemans, J. A. A. W. & Reek, J. N. H. Cofactor controlled encapsulation of a rhodium hydroformylation catalyst. Angew. Chem. Int. Ed. Engl. 58, 2696–2699 (2019).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Dydio, P., Dzik, W. I., Lutz, M., de Bruin, B. & Reek, J. N. H. Remote supramolecular control of catalyst selectivity in the hydroformylation of alkenes. Angew. Chem. Int. Ed. Engl. 50, 396–400 (2011).
Yoshioka, S., Inokuma, Y., Hoshino, M., Sato, T. & Fujita, M. Absolute structure determination of compounds with axial and planar chirality using the crystalline sponge method. Chem. Sci. 6, 3765–3768 (2015).
Cordier, P., Tournilhac, F., Soulié-Ziakovic, C. & Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 451, 977–980 (2008).
Sepehrpour, H., Fu, W., Sun, Y. & Stang, P. J. Biomedically relevant self-assembled metallacycles and metallacages. J. Am. Chem. Soc. 141, 14005–14020 (2019).
Dankers, P. Y. W. et al. The use of fibrous, supramolecular membranes and human tubular cells for renal epithelial tissue engineering: towards a suitable membrane for a bioartificial kidney. Macromol. Biosci. 10, 1345–1354 (2010).
Anelli, P. L. et al. Molecular meccano. 1. [2]Rotaxanes and a [2]catenane made to order. J. Am. Chem. Soc. 114, 193–218 (1992).
Bols, P. S. & Anderson, H. L. Template-directed synthesis of molecular nanorings and cages. Acc. Chem. Res. 51, 2083–2092 (2018).
Fuller, A.-M. L., Leigh, D. A. & Lusby, P. J. One template, multiple rings: controlled iterative addition of macrocycles onto a single binding site rotaxane thread. Angew. Chem. Int. Ed. Engl. 46, 5015–5019 (2007).
Meng, W. et al. A self-assembled M8L6 cubic cage that selectively encapsulates large aromatic guests. Angew. Chem. Int. Ed. Engl. 50, 3479–3483 (2011).
Yoshizawa, M., Klosterman, J. K. & Fujita, M. Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. Engl. 48, 3418–3438 (2009).
Sun, Q.-F., Murase, T., Sato, S. & Fujita, M. A sphere-in-sphere complex by orthogonal self-assembly. Angew. Chem. Int. Ed. Engl. 50, 10318–10321 (2011).
Clever, G. H. & Punt, P. Cation–anion arrangement patterns in self-assembled Pd2L4 and Pd4L8 coordination cages. Acc. Chem. Res. 50, 2233–2243 (2017).
Rao, S.-J., Zhang, Q., Ye, X.-H., Gao, C. & Qu, D.-H. Integrative self-sorting: one-pot synthesis of a hetero[4]rotaxane from a daisy-chain-containing hetero[4]pseudorotaxane. Chem. Asian J. 13, 815–821 (2018).
He, Z., Jiang, W. & Schalley, C. A. Integrative self-sorting: a versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 44, 779–789 (2015).
Rama, T. et al. Integrative self-sorting of bipyridinium/diazapyrenium-based ligands into pseudo[1]rotaxanes. Chem. Eur. J. 23, 16743–16747 (2017).
Fahrenbach, A. C., Bruns, C. J., Cao, D. & Stoddart, J. F. Ground-state thermodynamics of bistable redox-active donor–acceptor mechanically interlocked molecules. Acc. Chem. Res. 45, 1581–1592 (2012).
Datta, S., Saha, M. L. & Stang, P. J. Hierarchical assemblies of supramolecular coordination complexes. Acc. Chem. Res. 51, 2047–2063 (2018).
Wang, A., Huang, J. & Yan, Y. Hierarchical molecular self-assemblies: construction and advantages. Soft Matter 10, 3362–3373 (2014).
Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Mastering molecular matter. Supramolecular architectures by hierarchical self-assembly. J. Mater. Chem. 13, 2661–2670 (2003).
Chen, L. J. & Yang, H. B. Construction of stimuli-responsive functional materials via hierarchical self-assembly involving coordination interactions. Acc. Chem. Res. 51, 2699–2710 (2018).
Service, R. F. How far can we push chemical self-assembly? Science 309, 95–95 (2005).
Wang, F., Liao, R. & Wang, F. Pathway control of π‐conjugated supramolecular polymers by incorporating donor‐acceptor functionality. Angew. Chem. Int. Ed. Engl. 62, e202305827 (2023).
Borsdorf, L. et al. Pathway-controlled aqueous supramolecular polymerization via solvent-dependent chain conformation effects. J. Am. Chem. Soc. 145, 8882–8895 (2023).
Leira-Iglesias, J., Sorrenti, A., Sato, A., Dunne, P. A. & Hermans, T. M. Supramolecular pathway selection of perylenediimides mediated by chemical fuels. Chem. Commun. 52, 9009–9012 (2016).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Korevaar, P. A., De Greef, T. F. A. & Meijer, E. W. Pathway complexity in π-conjugated materials. Chem. Mat. 26, 576–586 (2014).
Baskakov, I. V., Legname, G., Baldwin, M. A., Prusiner, S. B. & Cohen, F. E. Pathway complexity of prion protein assembly into amyloid. J. Biol. Chem. 277, 21140–21148 (2002).
Stern, M., Jayaram, V. & Murugan, A. Shaping the topology of folding pathways in mechanical systems. Nat. Commun. 9, 4303 (2018).
Matern, J., Kartha, K. K., Sánchez, L. & Fernández, G. Consequences of hidden kinetic pathways on supramolecular polymerization. Chem. Sci. 11, 6780–6788 (2020).
Hori, A., Yamashita, K. I. & Fujita, M. Kinetic self-assembly: selective cross-catenation of two sterically differentiated PdII-coordination rings. Angew. Chem. Int. Ed. Engl. 43, 5016–5019 (2004).
Vantomme, G. & Meijer, E. W. The construction of supramolecular systems. Science 363, 1396–1397 (2019).
Bohne, C. Supramolecular dynamics. Chem. Soc. Rev. 43, 4037–4050 (2014).
Ran, N., Zhao, L., Chen, Z. & Tao, J. Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale. Green Chem. 10, 361–372 (2008).
Sheldon, R. A. E factors, green chemistry and catalysis: an odyssey. Chem.Commun. 39, 3352–3365 (2008).
Catalyst. In The IUPAC Compendium of Chemical Terminology (eds Kaiser, J. & Chalk, S.) (International Union of Pure and Applied Chemistry (IUPAC), 2019).
Rothenberg, G. Catalysis (Wiley-VHC Verlag GmbH & Co. KGaA, 2008).
van Leeuwen, P. W. N. M. Homogeneous Catalysis Understanding the Art (Kluwer Academic Publishers, 2004).
Busacca, C. A., Fandrick, D. R., Song, J. J. & Senanayake, C. H. in Applications of Transition Metal Catalysis in Drug Discovery and Development (eds Crawley, M. L. & Trost, B. M.) 1–24 (Wiley, 2012).
Knözinger, H. & Kochloefl, K. in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH Verlag GmbH & Co. KGaA, 2003).
Smil, V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (MIT Press, 2004).
Dub, P. A. & Gordon, J. C. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat. Rev. Chem. 2, 396–408 (2018).
Gimeno, N. & Vilar, R. Anions as templates in coordination and supramolecular chemistry. Coord. Chem. Rev. 250, 3161–3189 (2006).
Busch, D. H. In The Pedersen Memorial Issue, Advances in Inclusion Science Vol. 7 (eds Izatt, R. M. & Bradshaw, J. S.) 389–395 (Springer, 1992).
Anderson, S., Anderson, H. L. & Sanders, J. K. M. Expanding roles for templates in synthesis. Acc. Chem. Res. 26, 469–475 (1993).
Pedersen, C. J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 7017–7036 (1967).
Pedersen, C. J. The discovery of crown ethers (Noble Lecture). Angew. Chem. Int. Ed. Engl. 27, 1021–1027 (1988).
Rapenne, G., Dietrich-Buchecker, C. & Sauvage, J. P. Copper(I)- or iron(II)-templated synthesis of molecular knots containing two tetrahedral or octahedral coordination sites. J. Am. Chem. Soc. 121, 994–1001 (1999).
Sauvage, J.-P. From chemical topology to molecular machines (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 56, 11080–11093 (2017).
Asakawa, M. et al. Improved template-directed synthesis of cyclobis(paraquat-p-phenylene). J. Org. Chem. 61, 9591–9595 (1996).
Ashton, P. R. et al. Molecular meccano. 4. The self-assembly of [2]catenanes incorporating photoactive and electroactive π-extended systems. J. Am. Chem. Soc. 117, 11171–11197 (1995).
Ibukuro, F., Kusukawa, T. & Fujita, M. The guest-template syntheses of the host frameworks. J. Am. Chem. Soc. 120, 8561–8562 (1998).
Tateishi, T. et al. Navigated self-assembly of a Pd2L4 cage by modulation of an energy landscape under kinetic control. J. Am. Chem. Soc. 141, 19669–19676 (2020).
Poleschak, I., Kern, J.-M. & Sauvage, J.-P. A copper-complexed rotaxane in motion: pirouetting of the ring on the millisecond timescale. Chem. Commun. 4, 474–476 (2004).
Takahashi, S., Tateishi, T., Sasaki, Y., Sato, H. & Hiraoka, S. Towards kinetic control of coordination self-assembly: a case study of a Pd3L6 double-walled triangle to predict the outcomes by a reaction network model. Phys. Chem. Chem. Phys. 22, 26614–26626 (2020).
Furlan, R. L. E., Otto, S. & Sanders, J. K. M. Supramolecular templating in thermodynamically controlled synthesis. Proc. Natl Acad. Sci. USA 99, 4801–4804 (2002).
Loiseau, T. & Férey, G. Synthesis and X-ray structural characterization of a novel oxyfluorinated microporous gallium phosphate with encapsulated 1,4-diazabicyclo[2.2.2]octane as the template: Ga3(PO4)(HPO4)2F3(OH)C6N2H14·0.5H2O. J. Chem. Commun. 17, 1197–1198 (1992).
Day, V. W., Klemperer, W. G. & Yaghi, O. M. Synthesis and characterization of a soluble oxide inclusion complex, [CH3CN⊂(V12O324−)]. J. Am. Chem. Soc. 111, 5959–5961 (1989).
Sun, Q. F. et al. Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle ligand variation. Science 328, 1144–1147 (2010).
Harris, K., Fujita, D. & Fujita, M. Giant hollow MnL2n spherical complexes: structure, functionalisation and applications. Chem. Commun. 49, 6703–6712 (2013).
Fujita, D., Yokoyama, H., Ueda, Y., Sato, S. & Fujita, M. Geometrically restricted intermediates in the self-assembly of an M12L24 cuboctahedral complex. Angew. Chem. Int. Ed. Engl. 54, 155–158 (2015).
Kai, S., Shigeta, T., Kojima, T. & Hiraoka, S. Quantitative analysis of the self-assembly process of a Pd12L24 coordination sphere. Chem. Asian J. 12, 3203–3207 (2017).
Yoneya, M., Tsuzuki, S., Yamaguchi, T., Sato, S. & Fujita, M. Coordination-directed self-assembly of M12L24 nanocage: effects of kinetic trapping on the assembly process. ACS Nano 8, 1290–1296 (2014).
Poole, D. A., Bobylev, E. O., Mathew, S. & Reek, J. N. H. Topological prediction of palladium coordination cages. Chem. Sci. 11, 12350–12357 (2020).
Bobylev, E. O., Poole, D. A., Bruin, B. & Reek, J. N. H. How to prepare kinetically stable self‐assembled Pt12L24 nanocages while circumventing kinetic traps. Chem. Eur. J. 27, 12667–12674 (2021).
Bobylev, E., Poole, D., de Bruin, B. & Reek, J. N. H. Selective formation of Pt12L24 nanospheres by ligand design. Chem. Sci. 12, 7696–7705 (2021).
Sato, S., Ishido, Y. & Fujita, M. Remarkable stabilization of M12L24 spherical frameworks through the cooperation of 48 Pd(II)-pyridine interactions. J. Am. Chem. Soc. 131, 6064–6065 (2009).
Barnes, J. C., Juríček, M., Vermeulen, N. A., Dale, E. J. & Stoddart, J. F. Synthesis of ExnBox cyclophanes. J. Org. Chem. 78, 11962–11969 (2013).
Bruns, C. J. et al. Emergent ion-gated binding of cationic host–guest complexes within cationic M12L24 molecular flasks. J. Am. Chem. Soc. 136, 12027–12034 (2014).
Venturi, M., Dumas, S., Balzani, V., Cao, J. & Stoddart, J. F. Threading/dethreading processes in pseudorotaxanes. A thermodynamic and kinetic study. New J. Chem. 28, 1032–1037 (2004).
Castro, R., Nixon, K. R., Evanseck, J. D. & Kaifer, A. E. Effects of side arm length and structure of para-substituted phenyl derivatives on their binding to the host cyclobis(paraquat-p-phenylene). J. Org. Chem. 61, 7298–7303 (1996).
Poole, D. A. III, Bobylev, E. O., Mathew, S. & Reek, J. N. H. Entropy directs the self-assembly of supramolecular palladium coordination macrocycles and cages. Chem. Sci. 13, 10141–10148 (2022).
Activation energy. In The IUPAC Compendium of Chemical Terminology 5th edn (eds Kaiser, J. & Chalk, S.) Online version 5.0.0 (International Union of Pure and Applied Chemistry, 2025).
Li, D. et al. Viral‐capsid‐type vesicle‐like structures assembled from M12L24 metal–organic hybrid nanocages. Angew. Chem. Int. Ed. Engl. 50, 5182–5187 (2011).
Li, H., Luo, J. & Liu, T. Modification of the solution behavior of Pd12L24 metal–organic nanocages via PEGylation. Chem. Eur. J. 22, 17949–17952 (2016).
Liu, C. et al. Balancing ligand flexibility versus rigidity for the stepwise self‐assembly of M12L24 via M6L12 metal–organic cages. Chem. Eur. J. 26, 11960–11965 (2020).
MacCuspie, R. I. et al. Challenges for physical characterization of silver nanoparticles under pristine and environmentally relevant conditions. J. Environ. Monit. 13, 1212 (2011).
Farkas, N., Scaria, P. V., Woodle, M. C. & Dagata, J. A. Physical-chemical measurement method development for self-assembled, core-shell nanoparticles. Sci. Rep. 9, 1655 (2019).
Kikuchi, T., Sato, S., Fujita, D. & Fujita, M. Stepwise DNA condensation by a histone-mimic peptide-coated M12L24 spherical complex. Chem. Sci. 5, 3257 (2014).
Kamiya, N., Tominaga, M., Sato, S. & Fujita, M. Saccharide-coated M12L24 molecular spheres that form aggregates by multi-interaction with proteins. J. Am. Chem. Soc. 129, 3816–3817 (2007).
Sun, Q.-F., Sato, S. & Fujita, M. An M12(L1)12(L2)12 cantellated tetrahedron: a case study on mixed-ligand self-assembly. Angew. Chem. Int. Ed. Engl. 53, 13510–13513 (2014).
Reek. J. N. H. et al. NCHEM-22122509B_Reeketal_FIGSHARE.rar. Figshare https://doi.org/10.6084/m9.figshare.28163420.v1 (2025).
Acknowledgements
This study was supported by the Holland Research School for Molecular Sciences (HRSMC) and the University of Amsterdam. We acknowledge the University of Amsterdam for financial support to RPA sustainable chemistry. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank A. Ehlers for support performing the 1H NMR studies and E. Zuidinga for help carrying out the MS experiments.
Author information
Authors and Affiliations
Contributions
T.B., S.M. and J.N.H.R. proposed, designed and conceptualized the research. T.B. synthesized and characterized the ligands, the macrocycle and pseudorotaxane, together with S.M. All NMR experiments were performed by T.B. supported by D.A.P. and S.M. The ESI–MS measurements were performed and analysed by T.B. and E.O.B. The MD simulations were carried out and analysed by D.A.P. The DLS and AFM experiments were performed and analysed by S.M., L.S.D.A. and E.A.-L. Remaining experiments were designed by T.B., S.M. and J.N.H.R. Funding acquisition was by T.B. and J.N.H.R. and project administration was realized by T.B. and S.M. The supervisors during this project were S.M., E.A.-L. and J.N.H.R. The manuscript was prepared by T.B., S.M. and J.N.H.R. and revised with the input of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Yuya Domoto, Divya Nayar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Methods, experimental details, Supplementary Figs. 1–40 and Tables 1–5.
Source data
Source Data Fig. 2
Source data NMR.
Source Data Fig. 4
Source data NMR and MS.
Source Data Fig. 5
Source data DLS and AFM.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouwens, T., Bobylev, E.O., Antony, L.S.D. et al. Exo-templating via pseudorotaxane formation reduces pathway complexity in the multicomponent self-assembly of M12L24 nanospheres. Nat. Chem. 17, 1067–1075 (2025). https://doi.org/10.1038/s41557-025-01808-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01808-w