Abstract
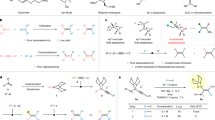
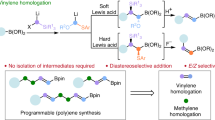
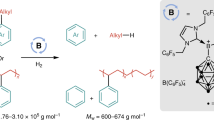
Organoboron compounds are important intermediates in organic synthesis, commonly used in metal-catalysed cross-coupling reactions. Their unique reactivity also allows modifications of their carbon framework with preservation of the valuable boryl group. Traditionally, these homologation reactions have been confined to the formation of alkyl boron compounds via C(sp3) insertion into a C–B bond. However, recent advancements in C(sp2)-insertive homologation highlight the potential of these reactions in synthesizing complex alkenes, despite current limitations in scope and control of the alkene geometry. Here we demonstrate a catalytic C(sp2)-insertive homologation for the regio- and diastereoselective synthesis of complex trisubstituted diborylalkenes from simple alkylboranes and alkynyl boronic esters. Our work demonstrates a broad reaction scope and application of the resulting products in modular and stereoselective synthesis of highly substituted alkenes. Furthermore, we provide evidence supporting a unique mechanism responsible for the excellent stereoselectivity observed in the reaction.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Crystallographic data for compound 29 are available free of charge from the Cambridge Crystallographic Data Centre under reference CCDC 2370487. All other data are available in the article or its Supplementary Information.
References
Lennox, A. J. J. & Lloyd-Jones, G. C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 43, 412–443 (2014).
Farfán-García, E. D. et al. Current data regarding the structure–toxicity relationship of boron-containing compounds. Toxicol. Lett. 258, 115–125 (2016).
Pagett, A. B. & Lloyd-Jones, G. C. in Organic Reactions (Wiley, 2019).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Go, S. Y. et al. A unified synthetic strategy to introduce heteroatoms via electrochemical functionalization of alkyl organoboron reagents. J. Am. Chem. Soc. 144, 9149–9160 (2022).
Xu, N., Liang, H. & Morken, J. P. Copper-catalyzed stereospecific transformations of alkylboronic esters. J. Am. Chem. Soc. 144, 11546–11552 (2022).
Mlynarski, S. N., Karns, A. S. & Morken, J. P. Direct stereospecific amination of alkyl and aryl pinacol boronates. J. Am. Chem. Soc. 134, 16449–16451 (2012).
Kabalka, G. W., Shoup, T. M. & Goudgaon, N. M. Sodium perborate: a mild and convenient reagent for efficiently oxidizing organoboranes. J. Org. Chem. 54, 5930–5933 (1989).
Kabalka, G. W. & Mereddy, A. R. Synthesis of organic bromides via organotrifluoroborates. Organometallics 23, 4519–4521 (2004).
Liang, H., Berwanger, M. R. & Morken, J. P. Stereospecific phosphination and thioetherification of organoboronic esters. J. Am. Chem. Soc. 146, 18873–18878 (2024).
Larouche-Gauthier, R., Elford, T. G. & Aggarwal, V. K. Ate complexes of secondary boronic esters as chiral organometallic-type nucleophiles for asymmetric synthesis. J. Am. Chem. Soc. 133, 16794–16797 (2011).
Matteson, D. S. & Majumdar, D. α-Chloro boronic esters from homologation of boronic esters. J. Am. Chem. Soc. 102, 7588–7590 (1980).
Matteson, D. S. & Majumdar, D. Homologation of boronic esters to α-chloro boronic esters. Organometallics 2, 1529–1535 (1983).
Matteson, D. S., Collins, B. S. L., Aggarwal, V. K. & Ciganek, E. The Matteson reaction. Org. React. 105, 427–860 (2021).
Matteson, D. S. & Ray, R. Directed chiral synthesis with pinanediol boronic esters. J. Am. Chem. Soc. 102, 7590–7591 (1980).
Matteson, D. S. α-Halo boronic esters in asymmetric synthesis. Tetrahedron 54, 10555–10607 (1998).
Stymiest, J. L., Bagutski, V., French, R. M. & Aggarwal, V. K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456, 778–782 (2008).
Stymiest, J. L., Dutheuil, G., Mahmood, A. & Aggarwal, V. K. Lithiated carbamates: chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 46, 7491–7494 (2007).
Casoni, G. et al. α-Sulfinyl benzoates as precursors to Li and Mg carbenoids for the stereoselective iterative homologation of boronic esters. J. Am. Chem. Soc. 139, 11877–11886 (2017).
Sharma, H. A., Essman, J. Z. & Jacobsen, E. N. Enantioselective catalytic 1,2-boronate rearrangements. Science 374, 752–757 (2021).
Zhang, L. et al. Catalytic conjunctive cross-coupling enabled by metal-induced metallate rearrangement. Science 351, 70–74 (2016).
Namirembe, S. & Morken, J. P. Reactions of organoboron compounds enabled by catalyst-promoted metalate shifts. Chem. Soc. Rev. 48, 3464–3474 (2019).
Aparece, M. D., Gao, C., Lovinger, G. J. & Morken, J. P. Vinylidenation of organoboronic esters enabled by a Pd-catalyzed metallate shift. Angew. Chem. Int. Ed. 58, 592–595 (2019).
Fordham, J. M., Grayson, M. N. & Aggarwal, V. K. Vinylidene homologation of boronic esters and its application to the synthesis of the proposed structure of machillene. Angew. Chem. Int. Ed. 58, 15268–15272 (2019).
Chen, M., Tugwell, T. H., Liu, P. & Dong, G. Synthesis of alkenyl boronates through stereoselective vinylene homologation of organoboronates. Nat. Synth. 3, 337–346 (2024).
Chen, M. et al. Stereospecific alkenylidene homologation of organoboronates by SNV reaction. Nature 631, 328–334 (2024).
Wang, H., Jing, C., Noble, A. & Aggarwal, V. K. Stereospecific 1,2-migrations of boronate complexes induced by electrophiles. Angew. Chem. Int. Ed. 59, 16859–16872 (2020).
Trost, B. The atom economy—a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Sheldon, R. A. Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev. 41, 1437–1451 (2012).
Charles, M. D., Schultz, P. & Buchwald, S. L. Efficient Pd-catalyzed amination of heteroaryl halides. Org. Lett. 7, 3965–3968 (2005).
Gillis, E. P. & Burke, M. D. A simple and modular strategy for small molecule synthesis: iterative Suzuki–Miyaura coupling of B-protected haloboronic acid building blocks. J. Am. Chem. Soc. 129, 6716–6717 (2007).
Li, J., Grillo, A. S. & Burke, M. D. From synthesis to function via iterative assembly of N-methyliminodiacetic acid boronate building blocks. Acc. Chem. Res. 48, 2297–2307 (2015).
Noguchi, H., Hojo, K. & Suginome, M. Boron-masking strategy for the selective synthesis of oligoarenes via iterative Suzuki–Miyaura coupling. J. Am. Chem. Soc. 129, 758–759 (2007).
Iwadate, N. & Suginome, M. Differentially protected diboron for regioselective diboration of alkynes: internal-selective cross-coupling of 1-alkene-1,2-diboronic acid derivatives. J. Am. Chem. Soc. 132, 2548–2549 (2010).
Peng, S., Liu, G. & Huang, Z. Mixed diboration of alkynes catalyzed by LiOH: regio- and stereoselective synthesis of cis-1,2-diborylalkenes. Org. Lett. 20, 7363–7366 (2018).
Kojima, C., Lee, K.-H., Lin, Z. & Yamashita, M. Direct and base-catalyzed diboration of alkynes using the unsymmetrical diborane(4), pinB-BMes2. J. Am. Chem. Soc. 138, 6662–6669 (2016).
Xu, L., Zhang, S. & Li, P. Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev. 44, 8848–8858 (2015).
Viso, A., Fernández, de la Pradilla, R. & Tortosa, M. Site-selective functionalization of C(sp3) vicinal boronic esters. ACS Catal. 12, 10603–10620 (2022).
Yang, C.-T., Zhang, Z.-Q., Liu, Y.-C. & Liu, L. Copper-catalyzed cross-coupling reaction of organoboron compounds with primary alkyl halides and pseudohalides. Angew. Chem. Int. Ed. 50, 3904–3907 (2011).
Molander, G. A. & Sandrock, D. L. Orthogonal reactivity in boryl-substituted organotrifluoroborates. J. Am. Chem. Soc. 130, 15792–15793 (2008).
Baumann, J. E. & Lalic, G. Differential dihydrofunctionalization: a dual catalytic three-component coupling of alkynes, alkenyl bromides, and pinacolborane. Angew. Chem. Int. Ed. 61, e202206462 (2022).
Hashimoto, T., Hatakeyama, T. & Nakamura, M. Stereospecific cross-coupling between alkenylboronates and alkyl halides catalyzed by iron–bisphosphine complexes. J. Org. Chem. 77, 1168–1173 (2012).
Thiede, S. et al. Regiodivergent iodocyclizations for the highly diastereoselective synthesis of syn- and anti-hydroxyl-isochromanones and -isobenzofuranones: concise synthesis of the isochromanone core of the ajudazols. Synthesis 48, 697–709 (2016).
Shimamoto, Y., Sunaba, H., Ishida, N. & Murakami, M. Regioselective construction of indene skeletons by palladium-catalyzed annulation of alkynylborates with o-iodophenyl ketones. Eur. J. Org. Chem. 2013, 1421–1424 (2013).
Lee, M. T. & Lalic, G. Mechanism of Z-selective hydroalkylation of terminal alkynes. J. Am. Chem. Soc. 143, 16663–16672 (2021).
Thiele, K. H., Engelhardt, G., Köhler, J. & Arnstedt, M. Beitrag zur Kenntnis von Allyl-Zink-Verbingungen II. Darstellung und Eigenschaften von Dimethallylzink und Dicrotylzink. J. Organomet. Chem. 9, 385–393 (1967).
Srebnik, M. Stereospecific preparation of trisubstituted allylic alcohols by alkene transfer from boron to zinc. Tetrahedron Lett. 32, 2449–2452 (1991).
Oppolzer, W. & Radinov, R. N. Catalytic asymmetric synthesis of secondary (E)-allyl alcohols from acetylenes and aldehydes via (1-alkenyl)zinc intermediates. Preliminary communication. Helv. Chim. Acta 75, 170–173 (1992).
Bart, S. C., Hawrelak, E. J., Schmisseur, A. K., Lobkovsky, E. & Chirik, P. J. Synthesis, reactivity, and solid state structures of four-coordinate iron(II) and manganese(II) alkyl complexes. Organometallics 23, 237–246 (2004).
Panne, P. & Fox, J. M. Rh-catalyzed intermolecular reactions of alkynes with α-diazoesters that possess β-hydrogens: ligand-based control over divergent pathways. J. Am. Chem. Soc. 129, 22–23 (2007).
Acknowledgements
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences grant no. R35GM158014 to G.L. and grant no. S10OD030224-01 to the University of Washington, Department of Chemistry NMR facility). We thank H. W. Kaminsky and S. M. Krajewski for determination of the X-ray crystal structure.
Author information
Authors and Affiliations
Contributions
B.W.G. conceived of, designed and performed the experiments. B.W.G. and G.L. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, experimental procedures, product characterization data and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 29; CCDC reference 2370487.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gardner, B.W., Lalic, G. Catalytic C(sp2) homologation of alkylboranes. Nat. Chem. 17, 1418–1424 (2025). https://doi.org/10.1038/s41557-025-01854-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01854-4