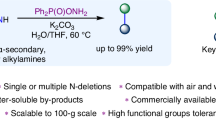
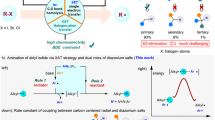
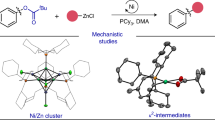
Abstract
Primary aliphatic amines are essential components in numerous functional molecules and rank among the most readily available commercial building blocks. Although commonly utilized as nitrogen nucleophiles, their application as alkyl sources for constructing (sp3)C–C(sp3) bonds remains a notable challenge. Here we present an approach integrating nitrogen-atom deletion into the aza-Michael reaction, thereby redirecting the classical pathway from (sp3)C–N bond formation to (sp3)C–C(sp3) bond construction. Leveraging commercially available O-diphenylphosphinylhydroxylamine as an efficient nitrogen-deletion reagent, this method enables a wide variety of primary aliphatic amines to serve as alkyl sources in couplings with structurally diverse electron-deficient olefins. This Giese-type reaction proceeds under mild conditions, achieves completion within 10 min and exhibits broad functional-group compatibility. By bridging two foundational transformations—the aza-Michael reaction and the Giese-type reaction—this approach interlinks their product spaces through a unified precursor library, substantially enhancing synthetic utility.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Ertl, P., Altmann, E. & McKenna, J. M. The most common functional groups in bioactive molecules and how their popularity has evolved over time. J. Med. Chem. 63, 8408–8418 (2020).
Lawrence, S. A. Amines: Synthesis, Properties and Applications (Cambridge Univ. Press, 2004).
Rulev, Y. A. Aza-Michael reaction: a decade later—is the research over? Eur. J. Org. Chem. 26, e202300451 (2023).
Zabolotna, Y. et al. A close-up look at the chemical space of commercially available building blocks for medicinal chemistry. J. Chem. Inf. Model. 62, 2171–2218 (2022).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Ouyang, K., Hao, W., Zhang, W.-X. & Xi, Z. Transition-metal-catalyzed cleavage of C–N single bonds. Chem. Rev. 115, 12045–12090 (2015).
Wang, Q., Su, Y., Li, L. & Huang, H. Transition-metal catalysed C–N bond activation. Chem. Soc. Rev. 45, 1257–1272 (2016).
Kong, D., Moon, P. J. & Lundgren, R. J. Radical coupling from alkyl amines. Nat. Catal. 2, 473–476 (2019).
Davey, S. Activating amines. Nat. Chem. https://doi.org/10.1038/nchem.867 (2010).
Li, Y., Xiao, F., Guo, Y. & Zeng, Y. Recent developments in deaminative functionalization of alkyl amines. Eur. J. Org. Chem. 8, 1215–1228 (2021).
Berger, K. J. et al. Direct deamination of primary amines via isodiazene intermediates. J. Am. Chem. Soc. 143, 17366–17373 (2021).
Dherange, B. D. et al. Direct deaminative functionalization. J. Am. Chem. Soc. 145, 17–24 (2023).
Xue, J.-H. et al. Deaminative bromination, chlorination, and iodination of primary amines. iScience 26, 106255 (2023).
Xue, J.-H., Li, Y., Liu, Y., Li, Q. & Wang, H. Site-specific deaminative trifluoromethylation of aliphatic primary amines. Angew. Chem. Int. Ed. 63, e202319030 (2024).
Kim, M. et al. Accessing sulfonamides via formal SO2 insertion into C–N bonds. Nat. Chem. https://doi.org/10.1038/s41557-025-01848-2 (2025).
Ma, P., Guo, T. & Lu, H. Hydro- and deutero-deamination of primary amines using O-diphenylphosphinylhydroxylamine. Nat. Commun. 15, 10190 (2024).
Narayanam, J. M., Tucker, J. W. & Stephenson, C. R. Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 131, 8756–8757 (2009).
Nguyen, J. D., D’Amato, E. M., Narayanam, J. M. R. & Stephenson, C. R. J. Engaging unactivated alkyl, alkenyl and aryl iodides in visible-light-mediated free radical reactions. Nat. Chem. 4, 854–859 (2012).
Constantin, T. et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science 367, 1021–1026 (2020).
Kitcatt, D., Nicolle, S. & Lee, A. L. Direct decarboxylative Giese reactions. Chem. Soc. Rev. 51, 1415–1453 (2022).
Wang, J. Z., Sakai, H. A. & MacMillan, D. W. C. Alcohols as alkylating agents: photoredox-catalyzed conjugate alkylation via in situ deoxygenation. Angew. Chem. Int. Ed. 61, e202207150 (2022).
Gualandi, A. et al. Photocatalytic radical alkylation of electrophilic olefins by benzylic and alkylic zinc-sulfinates. ACS Catal. 7, 5357–5362 (2017).
Giese, B., González-Gómez, J. A. & Witzel, T. The scope of radical CC-coupling by the “tin method”. Angew. Chem. Int. Ed. 23, 69–70 (1984).
Juliá, F., Constantin, T. & Leonori, D. Applications of halogen-atom transfer (XAT) for the generation of carbon radicals in synthetic photochemistry and photocatalysis. Chem. Rev. 122, 2292–2352 (2022).
Gant Kanegusuku, A. L. & Roizen, J. L. Recent advances in photoredox-mediated radical conjugate addition reactions: an expanding toolkit for the Giese reaction. Angew. Chem. Int. Ed. 60, 21116–21149 (2021).
Gao, Y. et al. Recent progress in fragmentation of Katritzky salts enabling formation of C–C, C–B, and C–S bonds. Top. Curr. Chem. 380, 25–87 (2022).
Correia, J. T. M. et al. Photoinduced deaminative strategies: Katritzky salts as alkyl radical precursors. Chem. Commun. 56, 503–514 (2020).
Wu, J., Grant, P. S., Li, X., Noble, A. & Aggarwal, V. K. Catalyst-free deaminative functionalizations of primary amines by photoinduced single-electron transfer. Angew. Chem. Int. Ed. 58, 5697–5701 (2019).
Rössler, S. L. et al. Pyridinium salts as redox-active functional group transfer reagents. Angew. Chem. Int. Ed. 59, 9264–9280 (2020).
Ashley, M. A. & Rovis, T. Photoredox-catalyzed deaminative alkylation via C–N bond activation of primary amines. J. Am. Chem. Soc. 142, 18310–18316 (2020).
Dorsheimer, J. R., Ashley, M. A. & Rovis, T. Dual nickel/photoredox-catalyzed deaminative cross-coupling of sterically hindered primary amines. J. Am. Chem. Soc. 143, 19294–19299 (2021).
Dorsheimer, J. R. & Rovis, T. Late-stage isotopic exchange of primary amines. J. Am. Chem. Soc. 145, 24367–24374 (2023).
Marchese, A. D., Dorsheimer, J. R. & Rovis, T. Photoredox-catalyzed generation of tertiary anions from primary amines via a radical polar crossover. Angew. Chem. Int. Ed. 63, e202317563 (2024).
Quirós, I. et al. Isonitriles as alkyl radical precursors in visible light mediated hydro- and deuterodeamination reactions. Angew. Chem. Int. Ed. 63, e202317683 (2024).
Ma, Y.-Q., Zhang, M. & Tian, S.-K. Silyl radical as an isocyanide transfer agent for Giese-type reactions involving aliphatic amines. Org. Lett. 26, 5172–5176 (2024).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Li, E.-Q., Lindsley, C. W., Chang, J. & Yu, B. Molecular skeleton editing for new drug discovery. J. Med. Chem. 67, 13509–13511 (2024).
Unsworth, W. P. & Avestro, A.-J. Nitrogen deletion offers fresh strategy for organic synthesis. Nature 593, 203–204 (2021).
Huang, B. & Lu, H. N-atom deletion involving rearrangement of sulfamoyl azides or triazanium salts. Acc. Chem. Res. 58, 919–932 (2025).
Zippel, C., Seibert, J. & Bräse, S. Skeletal editing—nitrogen deletion of secondary amines by anomeric amide reagents. Angew. Chem. Int. Ed. 60, 19522–19524 (2021).
Shimazumi, R. & Tobisu, M. Unimolecular fragment coupling: a new bond-forming methodology via the deletion of atom(s). JACS Au 4, 1676–1695 (2024).
Zou, X., Zou, J., Yang, L., Li, G. & Lu, H. Thermal rearrangement of sulfamoyl azides: reactivity and mechanistic study. J. Org. Chem. 82, 4677–4688 (2017).
Qin, H. et al. N-atom deletion in nitrogen heterocycles. Angew. Chem. Int. Ed. 60, 20678–20683 (2021).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Wright, B. A. et al. Skeletal editing approach to bridge-functionalized bicyclo[1.1.1]pentanes from aza-bicyclo[2.1.1]hexanes. J. Am. Chem. Soc. 145, 10960–10966 (2023).
Holovach, S. et al. C–C coupling through nitrogen deletion: application to library synthesis. Chem. Eur. J. 29, e202203470 (2023).
Masson-Makdissi, J. et al. Evidence for dearomatizing spirocyclization and dynamic effects in the quasi-stereospecific nitrogen deletion of tetrahydroisoquinolines. J. Am. Chem. Soc. 146, 17719–17727 (2024).
Guo, T., Li, J., Cui, Z., Wang, Z. & Lu, H. C(sp3)–C(sp3) bond formation through nitrogen deletion of secondary amines using O-diphenylphosphinylhydroxylamine. Nat. Synth. 3, 913–921 (2024).
Hui, C., Brieger, L., Strohmann, C. & Antonchick, A. P. Stereoselective synthesis of cyclobutanes by contraction of pyrrolidines. J. Am. Chem. Soc. 143, 18864–18870 (2021).
Onnuch, P., Ramagonolla, K. & Liu, R. Y. Aminative Suzuki–Miyaura coupling. Science 383, 1019–1024 (2024).
Rieder, C. J. & Smith, M. V. An unexpected incident during the manufacture of O-(diphenylphosphinyl)hydroxylamine. Org. Process Res. Dev. 25, 2308–2314 (2021).
Tsien, J., Hu, C., Merchant, R. R. & Qin, T. Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. Nat. Rev. Chem. 8, 605–627 (2024).
Yasukawa, T., Håheim, K. S. & Cossy, J. Synthesis of 1,3-disubstituted bicyclo[1.1.1]pentanes by cross-coupling induced by transition metals—formation of C–C bonds. Org. Biomol. Chem. 21, 7666–7680 (2023).
Levin, M. D., Kaszynski, P. & Michl, J. Bicyclo[1.1.1]pentanes, [n]staffanes, [1.1.1]propellanes, and tricyclo[2.1.0.02,5]pentanes. Chem. Rev. 100, 169–234 (2000).
Marson, C. M.; Savy, P. in Comprehensive Organic Functional Group Transformation Vol. II (ed. Ramsden, C. A.) 255 (Elsevier, 2004).
Shioiri, T., Ninomiya, K. & Yamada, S. Diphenylphosphoryl azide. New convenient reagent for a modified Curtius reaction and for peptide synthesis. J. Am. Chem. Soc. 94, 6203–6205 (1972).
Norman, A. R., Yousif, M. N. & McErlean, C. S. P. Photoredox-catalyzed indirect acyl radical generation from thioesters. Org. Chem. Front. 5, 3267–3298 (2018).
Dutta, S., Li, B., Rickertsen, D. R. L., Valles, D. A. & Seidel, D. C–H bond functionalization of amines: a graphical overview of diverse methods. SynOpen 5, 173–228 (2021).
Chen, W., Cao, X. & Yang, X. Transition-metal-free methods for the remote C–H bond functionalization of cyclic amines. Asian J. Org. Chem. 12, e202200547 (2023).
Meng, X., Dong, Y., Liu, Q. & Wang, W. Organophotocatalytic α-deuteration of unprotected primary amines via H/D exchange with D2O. Chem. Commun. 60, 296–299 (2024).
Ryder, A. S. H. et al. Photocatalytic α-tertiary amine synthesis via C–H alkylation of unmasked primary amines. Angew. Chem. Int. Ed. 59, 14986–14991 (2020).
Liu, Y. & Ge, H. Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem. 9, 26–32 (2017).
Chen, Y.-Q. et al. Overcoming the limitations of γ- and δ-C–H arylation of Amines through ligand development. J. Am. Chem. Soc. 140, 17884–17894 (2018).
Xu, Y., Young, M. C., Wang, C., Magness, D. M. & Dong, G. Catalytic C(sp3)−H arylation of free primary amines with an exo directing group generated in situ. Angew. Chem. Int. Ed. 55, 9084–9087 (2016).
Wang, H., Tong, H.-R., He, G. & Chen, G. An enantioselective bidentate auxiliary directed palladium-catalyzed benzylic C–H arylation of amines using a BINOL phosphate ligand. Angew. Chem. Int. Ed. 55, 15387–15397 (2016).
Zhang, S.-Y. et al. Efficient alkyl ether synthesis via palladium-catalyzed, picolinamide-directed alkoxylation of unactivated C(sp3)–H and C(sp2)–H bonds at remote positions. J. Am. Chem. Soc. 134, 7313–7316 (2012).
Newcomb, M. in Encyclopedia of Radicals in Chemistry, Biology and Materials (eds Chatgilialoglu, C. & Studer, A.) (Wiley, 2012).
Qin, H., Guo, T., Lin, K., Li, G. & Lu, H. Synthesis of dienes from pyrrolidines using skeletal modification. Nat. Commun. 14, 7307 (2023).
Steiniger, K. A., Lamb, M. C. & Lambert, T. H. Cross-coupling of amines via photocatalytic denitrogenation of in situ generated diazenes. J. Am. Chem. Soc. 145, 11524–11529 (2023).
Acknowledgements
Financial support for this work was provided by the National Natural Science Foundation of China (22071100, 22271148) and the Natural Science Foundation of Jiangsu Province (BK20231400).
Author information
Authors and Affiliations
Contributions
P.M. and H.L. designed the experiments. P.M. and Z.C. performed the experiments and analysed the data. All authors participated in writing the paper. H.L. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Shi-Kai Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Experimental procedures and NMR spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, P., Cui, Z. & Lu, H. Deaminative Giese-type reaction. Nat. Chem. 17, 1556–1564 (2025). https://doi.org/10.1038/s41557-025-01888-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01888-8