Abstract
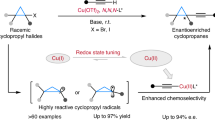
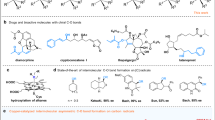
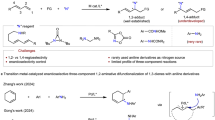
Achieving high enantioselectivity in asymmetric catalysis, especially with very reactive species such as radicals, often comes at the expense of generality. Radicals with exceptionally high reactivity are typically unsuitable for existing asymmetric methodologies. Here we present a general catalytic approach to asymmetric radical cross-coupling that combines copper-catalysed enantioselective stereocentre resolution or formation with copper-mediated, chirality-transferring radical substitution. This sequential strategy enables the efficient coupling of over 50 distinct carbon-, nitrogen-, oxygen-, sulfur- and phosphorus-centred radicals, including highly reactive methyl, tert-butoxyl and phenyl radicals, yielding structurally diverse C-, P- and S-chiral compounds with outstanding enantioselectivity. Our method thus provides a unified platform for the synthesis of carbon, phosphorus and sulfur stereocentres, with important implications for the preparation of chiral molecules relevant to medicinal chemistry and related disciplines. Furthermore, this sequential stereodiscrimination and chirality transfer strategy offers a promising blueprint for the development of highly enantioselective methodologies applicable to other classes of highly reactive species beyond radicals.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information (experimental procedures, characterization data and DFT calculations). Crystallographic data are also available free of charge from the Cambridge Crystallographic Database Centre (CCDC) under CCDC reference numbers 2289793 (for M1), 2375977 (for (R)-S5), 2375978 (for 9), 2375976 (for 20), 2289792 (for 36), 2289795 (for 46) and 2289794 (for 57). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Kürti, L. & Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms (Elsevier, 2005).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 5, 597–601 (2013).
Wagen, C. C., McMinn, S. E., Kwan, E. E. & Jacobsen, E. N. Screening for generality in asymmetric catalysis. Nature 610, 680–686 (2022).
Rein, J. et al. Generality-oriented optimization of enantioselective aminoxyl radical catalysis. Science 380, 706–712 (2023).
Proctor, R. S. J., Colgan, A. C. & Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 12, 990–1004 (2020).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Lee, W.-C. C. & Zhang, X. P. Metalloradical catalysis: general approach for controlling reactivity and selectivity of homolytic radical reactions. Angew. Chem. Int. Ed. 63, e202320243 (2024).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Zhang, H. et al. Site- and enantioselective allylic and propargylic C–H oxidation enabled by copper-based biomimetic catalysis. Nat. Catal. 8, 58–66 (2025).
Hioe, J. & Zipse, H. in Encyclopedia of Radicals in Chemistry, Biology and Materials (eds Chatgilialoglu, C. & Studer, A.) https://doi.org/10.1002/9781119953678.rad012 (Wiley, 2012).
Li, Y. et al. Cobalt-catalysed enantioselective C(sp3)–C(sp3) coupling. Nat. Catal. 4, 901–911 (2021).
Li, J. et al. Enantioselective alkylation of α-amino C(sp3)−H bonds via photoredox and nickel catalysis. Nat. Catal. 7, 889–899 (2024).
Upton, C. J. & Incremona, J. H. Bimolecular homolytic substitution at carbon. Stereochemical investigation. J. Org. Chem. 41, 523–530 (1976).
Walton, J. C. Homolytic substitution: a molecular ménage à trois. Acc. Chem. Res. 31, 99–107 (1998).
Zhang, Y., Li, K.-D. & Huang, H.-M. Bimolecular homolytic substitution (SH2) at a transition metal. ChemCatChem 16, e202400955 (2024).
Milan, M., Bietti, M. & Costas, M. Enantioselective aliphatic C–H bond oxidation catalyzed by bioinspired complexes. Chem. Commun. 54, 9559–9570 (2018).
Liu, W., Lavagnino, M. N., Gould, C. A., Alcázar, J. & MacMillan, D. W. C. A biomimetic SH2 cross-coupling mechanism for quaternary sp3-carbon formation. Science 374, 1258–1263 (2021).
Li, L.-J. et al. Enantioselective construction of quaternary stereocenters via cooperative photoredox/Fe/chiral primary amine triple catalysis. J. Am. Chem. Soc. 146, 9404–9412 (2024).
Wang, Y., Wen, X., Cui, X. & Zhang, X. P. Enantioselective radical cyclization for construction of 5-membered ring structures by metalloradical C–H alkylation. J. Am. Chem. Soc. 140, 4792–4796 (2018).
Lee, W.-C. C., Wang, D.-S., Zhu, Y. & Zhang, X. P. Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism. Nat. Chem. 15, 1569–1580 (2023).
Wang, F.-L. et al. Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes. Nat. Chem. 14, 949–957 (2022).
Lang, K., Torker, S., Wojtas, L. & Zhang, X. P. Asymmetric induction and enantiodivergence in catalytic radical C–H amination via enantiodifferentiative H-atom abstraction and stereoretentive radical substitution. J. Am. Chem. Soc. 141, 12388–12396 (2019).
Lang, K., Li, C., Kim, I. & Zhang, X. P. Enantioconvergent amination of racemic tertiary C–H bonds. J. Am. Chem. Soc. 142, 20902–20911 (2020).
Xu, P., Xie, J., Wang, D.-S. & Zhang, X. P. Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination. Nat. Chem. 15, 498–507 (2023).
Xu, H., Wang, D.-S., Zhu, Z., Deb, A. & Zhang, X. P. New mode of asymmetric induction for enantioselective radical N-heterobicyclization via kinetically stable chiral radical center. Chem 10, 283–298 (2024).
Tian, Y. et al. A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution. Nat. Chem. 16, 466–475 (2024).
Zhang, Y.-F. et al. Asymmetric amination of alkyl radicals with two minimally different alkyl substituents. Science 388, 283–291 (2025).
Cheng, Y.-F. et al. Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling. Nat. Chem. 15, 395–404 (2023).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Omann, L., Königs, C. D. F., Klare, H. F. T. & Oestreich, M. Cooperative catalysis at metal–sulfur bonds. Acc. Chem. Res. 50, 1258–1269 (2017).
Crich, D. Homolytic substitution at the sulfur atom as a tool for organic synthesis. Helv. Chim. Acta 89, 2167–2182 (2006).
Garrido-Castro, A. F., Salaverri, N., Maestro, M. C. & Alemán, J. Intramolecular homolytic substitution enabled by photoredox catalysis: sulfur, phosphorus, and silicon heterocycle synthesis from aryl halides. Org. Lett. 21, 5295–5300 (2019).
Coulomb, J. et al. Intramolecular homolytic substitution of sulfinates and sulfinamides. Chem. Eur. J. 15, 10225–10232 (2009).
Zhong, W. et al. Synthesis and reactivity of the imido-bridged metallothiocarboranes CpCo(S2C2B10H10)(NSO2R). Organometallics 31, 6658–6668 (2012).
Chen, J.-J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
van Dijkman, T. F. et al. Extremely bulky copper(I) complexes of [HB(3,5-{1-naphthyl}2pz)3]− and [HB(3,5-{2-naphthyl}2pz)3]− and their self-assembly on graphene. Dalton Trans. 46, 6433–6446 (2017).
Grotewold, J., Lissi, E. A. & Scaiano, J. C. Mechanism of the autoxidation of triethylborane. Part I. Reaction in the gas phase. J. Chem. Soc. B https://doi.org/10.1039/J29690000475 (1969).
Cai, A., Yan, W., Wang, C. & Liu, W. Copper-catalyzed difluoromethylation of alkyl iodides enabled by aryl radical activation of carbon–iodine bonds. Angew. Chem. Int. Ed. 60, 27070–27077 (2021).
Luo, Y.-R. Comprehensive Handbook of Chemcial Bond Energies (CRC Press, 2007).
Parsaee, F. et al. Radical philicity and its role in selective organic transformations. Nat. Rev. Chem. 5, 486–499 (2021).
Garwood, J. J. A., Chen, A. D. & Nagib, D. A. Radical polarity. J. Am. Chem. Soc. 146, 28034–28059 (2024).
Tsentalovich, Y. P., Kulik, L. V., Gritsan, N. P. & Yurkovskaya, A. V. Solvent effect on the rate of β-scission of the tert-butoxyl radical. J. Phys. Chem. A 102, 7975–7980 (1998).
Zhang, Z.-X. & Willis, M. C. Sulfondiimidamides as new functional groups for synthetic and medicinal chemistry. Chem 8, 1137–1146 (2022).
Zhang, X., Wang, F. & Tan, C.-H. Asymmetric synthesis of S(IV) and S(VI) stereogenic centers. JACS Au 3, 700–714 (2023).
Zhang, X., Ang, E. C. X., Yang, Z., Kee, C. W. & Tan, C.-H. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022).
Greenwood, N. S., Champlin, A. T. & Ellman, J. A. Catalytic enantioselective sulfur alkylation of sulfenamides for the asymmetric synthesis of sulfoximines. J. Am. Chem. Soc. 144, 17808–17814 (2022).
van Dijk, L. et al. Data science-enabled palladium-catalyzed enantioselective aryl-carbonylation of sulfonimidamides. J. Am. Chem. Soc. 145, 20959–20967 (2023).
Liang, Q. et al. Enantioselective Chan–Lam S-arylation of sulfenamides. Nat. Catal. 7, 1010–1020 (2024).
Champlin, A. T., Kwon, N. Y. & Ellman, J. A. Enantioselective S-alkylation of sulfenamides by phase-transfer catalysis. Angew. Chem. Int. Ed. 63, e202408820 (2024).
Robak, M. T., Herbage, M. A. & Ellman, J. A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 110, 3600–3740 (2010).
Tsuzuki, S. & Kano, T. Asymmetric synthesis of chiral sulfimides through the O-alkylation of enantioenriched sulfinamides and addition of carbon nucleophiles. Angew. Chem. Int. Ed. 62, e202300637 (2023).
Homer, J. A. et al. Sulfur fluoride exchange. Nat. Rev. Methods Primers 3, 58 (2023).
Acknowledgements
We appreciate the assistance of SUSTech Core Research Facilities. We acknowledge the financial support from the National Natural Science Foundation of China (grant nos. 22025103, 92256301 and 22331006 to X.-Y.L., 22271133 to Q.-S.G., and W2512004, 22122109 and 22271253 to X.H.), the National Key R&D Program of China (grant nos. 2021YFF0701604 to X.-Y.L., 2022YFC3401104 to Z.D. and 2022YFA1504301 to X.H.), Guangdong Innovative Program (grant nos. 2019BT02Y335 to X.-Y.L. and 2021ZT09C278 to Z.D.), the Guangdong Major Project of Basic and Applied Basic Research (grant no. 2023B0303000020 to X.-Y.L. and Z.D.), Shenzhen Science and Technology Program (grant nos. KQTD20210811090112004 to X.-Y.L. and Q.-S.G., JCYJ20220818100600001 to X.-Y.L., JCYJ20220818100604009 to J.-B.T. and 20220814231741002 to Z.D.), the New Cornerstone Science Foundation through an XPLORER PRIZE (to X.-Y.L.), High-Level of Special Funds (grant no. G03050K003 to X.-Y.L.), High-Level Key Discipline Construction Project (grant no. G030210001 to X.-Y.L. and Q.-S.G.), the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (grant no. SN-ZJU-SIAS-006 to X.H.), CAS Youth Interdisciplinary Team (grant no. JCTD-2021-11 to X.H.), the Fundamental Research Funds for the Central Universities (grant nos. 226-2022-00140, 226-2022-00224, 226-2023-00115 and 226-2024-00003 to X.H), the State Key Laboratory of Clean Energy Utilization (grant no. ZJUCEU2020007 to X.H.), the State Key Laboratory of Physical Chemistry of Solid Surfaces (grant no. 202210 to X.H.), a Leading Innovation Team grant from the Department of Science and Technology of Zhejiang Province (grant no. 2022R01005 to X.H.) and the Open Research Fund of the School of Chemistry and Chemical Engineering of Henan Normal University (grant no. 2024Z01 to X.H.). Computational studies were supported by the Center for Computational Science and Engineering of Southern University of Science and Technology and the high-performance computing system at the Department of Chemistry, Zhejiang University.
Author information
Authors and Affiliations
Contributions
L.-W.F., J.-B.T., L.-L.W. and Z.G. designed the experiments and analysed the data. L.-W.F., J.-B.T., L.-L.W., Z.G., Y.-S.Z., D.-L.Y., L.Q., Y.T., Z.-C.C., F.L., J.-M.X., P.-J.H., W.-L.L., C.-Y.X., C.L. and Z.-L.L. performed the experiments. J.-R.L. and X.H. designed the DFT calculations, and J.-R.L. performed the calculations. X.H., Z.D., Q.-S.G. and X.-Y.L. wrote the paper. Z.D., Q.-S.G. and X.-Y.L. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, Tables 1–22, Experimental procedures, Synthetic procedures, Characterization data, Density functional theory calculations and Mechanistic discussion.
Supplementary Data
Cartesian coordinates of computed species.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, LW., Tang, JB., Wang, LL. et al. Copper-catalysed asymmetric cross-coupling reactions tolerant of highly reactive radicals. Nat. Chem. 18, 142–151 (2026). https://doi.org/10.1038/s41557-025-01970-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01970-1