Abstract
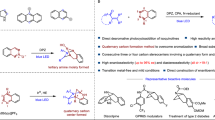
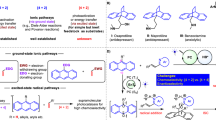
The formation of electron donor–acceptor (EDA) complexes has emerged as a powerful strategy for accessing valuable molecules. However, protocols for constructing enantio-enriched molecules are limited, typically relying on EDA complex formation between a substrate and an intermediate generated from a chiral catalyst along with another substrate. This approach facilitates the coupling of the resulting radical species, thereby enabling the controlled enantioselective formation of stereocentres. Here we introduce a kinetic resolution strategy that harnesses the formation of EDA complexes between a chiral catalyst and racemic feedstock. The key lies in the thermodynamic disparity of the chiral catalyst’s interaction with the two enantiomers of the substrate, which is critical for enabling subsequent transformations under a kinetic control. We demonstrate that photochemical reactions involving racemic azaarene-functionalized tertiary alcohols and amines, together with a chiral phosphoric acid, yield enantio-enriched derivatives containing tertiary carbon stereocentres. The utility of this approach is further exemplified by synthesizing important azaarene variants featuring tertiary or secondary C–F bonds, as well as ethylene oxides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available in the paper and its Supplementary Information. Crystallographic data for the structure of 1l have been deposited at the Cambridge Crystallographic Data Centre, under deposition no. CCDC 2416697. A copy of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
References
Mulliken, R. S. Structures of complexes formed by halogen molecules with aromatic and with oxygenated solvents. J. Am. Chem. Soc. 72, 600–608 (1950).
Mulliken, R. S. Molecular compounds and their spectra. III. The interaction of electron donors and acceptors. J. Phys. Chem. 56, 801–822 (1952).
Mulliken, R. S. Molecular compounds and their spectra. II. J. Am. Chem. Soc. 74, 811–824 (1952).
Lima, C. G. S. et al. Organic synthesis enabled by light-irradiation of EDA complexes: theoretical background and synthetic applications. ACS Catal. 6, 1389–1407 (2016).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Wang, P.-Z., Xiao, W.-J. & Chen, J.-R. Light-empowered contra-thermodynamic stereochemical editing. Nat. Rev. Chem. 7, 35–50 (2023).
Wortman, A. K. & Stephenson, C. R. J. EDA photochemistry: mechanistic investigations and future opportunities. Chem 9, 2390–2415 (2023).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5322 (2013).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Arceo, E., Jurberg, I. D., Álvarez-Fernández, A. & Melchiorre, P. Photochemical activity of a key donor–acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat. Chem. 5, 750–756 (2013).
Arceo, E., Bahamonde, A., Bergonzini, G. & Melchiorre, P. Enantioselective direct α-alkylation of cyclic ketones by means of photo-organocatalysis. Chem. Sci. 5, 2438–2442 (2014).
Bahamonde, A. & Melchiorre, P. Mechanism of the stereoselective α-alkylation of aldehydes driven by the photochemical activity of enamines. J. Am. Chem. Soc. 138, 8019–8030 (2016).
Cao, Z.-Y., Ghosh, T. & Melchiorre, P. Enantioselective radical conjugate additions driven by a photoactive intramolecular iminium-ion-based EDA complex. Nat. Commun. 9, 3274 (2018).
Woźniak, Ł, Murphy, J. J. & Melchiorre, P. Photo-organocatalytic enantioselective perfluoroalkylation of β-ketoesters. J. Am. Chem. Soc. 137, 5678–5681 (2015).
Bauer, A., Westkämper, F., Grimme, S. & Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436, 1139–1140 (2005).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Huo, H. et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 515, 100–103 (2014).
Blum, T. R. et al. Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer. Science 354, 1391–1395 (2016).
Yao, W., Bazan-Bergamino, E. A. & Ngai, M.-Y. Asymmetric photocatalysis enabled by chiral organocatalysts. ChemCatChem 14, e202101292 (2022).
Hölzl-Hobmeier, A. et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564, 240–243 (2018).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Bønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Cussac, M., Boucherle, A., Pierre, J. L. & Hache, J. Pyridyl ketones of biological interest (chalcone analogs). VI. Alcohol derivatives of azachalcones with depressant activity on the central nervous system. Eur. J. Med. Chem. 9, 651–657 (1974).
Lin, T. K. et al. Quantum statistical calculation for the correlation of biological activity and chemical structure. 3. Disopyramide phosphate and related compounds as antiarrhythmic agents. J. Med. Chem. 17, 751–753 (1974).
Yen, C. H., Lowrie, H. S. & Dean, R. R. Compounds related to 4-diisopropylamino-2-phenyl-2-(2-pyridyl)butyramide. Synthesis and antiarrhythmic activity. J. Med. Chem. 17, 1131–1135 (1974).
Rennison, D. et al. Synthesis and activity studies of analogues of the rat selective toxicant norbormide. Bioorg. Med. Chem. 15, 2963–2974 (2007).
Rehdorf, J. et al. Pseudomonas putida esterase contains A GGG(A)X-motif confering activity for the kinetic resolution of tertiary alcohols. Appl. Microbio. Biotech. 93, 1119–1126 (2012).
Harikrishnan, L. S. et al. Diphenylpyridylethanamine (DPPE) derivatives as cholesteryl ester transfer protein (CETP) inhibitors. J. Med. Chem. 55, 6162–6175 (2012).
Huang, A. et al. Discovery of 2-[1-(4-chlorophenyl)cyclopropyl]-3-hydroxy-8-(trifluoromethyl)quinoline-4-carboxylic acid (PSI-421), a P-Selectin inhibitor with improved pharmacokinetic properties and oral efficacy in models of vascular injury. J. Med. Chem. 53, 6003–6017 (2010).
Hou, L. et al. Enantioselective radical addition to ketones through Lewis acid-enabled photoredox catalysis. J. Am. Chem. Soc. 144, 22140–22149 (2022).
Yin, Y., Zhao, X. & Jiang, Z. Asymmetric photocatalytic synthesis of enantioenriched azaarene derivatives. Chin. J. Org. Chem. 42, 1609–1625 (2022).
Yin, Y. et al. Conjugate addition–enantioselective protonation of N-aryl glycines to α-branched 2-vinylazaarenes via cooperative photoredox and asymmetric catalysis. J. Am. Chem. Soc. 140, 6083–6087 (2018).
Liu, Y. et al. Asymmetric olefin isomerization via photoredox catalytic hydrogen atom transfer and enantioselective protonation. J. Am. Chem. Soc. 145, 18307–18315 (2023).
Ma, C. et al. Enantioselective chemodivergent three-component radical tandem reactions through asymmetric photoredox catalysis. J. Am. Chem. Soc. 145, 20141–20148 (2023).
Fu, Q. et al. Enantioselective [2π + 2σ] cycloadditions of bicyclo[1.1.0]butanes with vinylazaarenes through asymmetric photoredox catalysis. J. Am. Chem. Soc. 146, 8372–8380 (2024).
Sun, X. et al. Asymmetric photoredox catalytic formal De Mayo reaction enabled by sensitization-initiated electron transfer. Nat. Chem. 16, 1169–1176 (2024).
Wang, J. et al. Enantioselective [2 + 2] photocycloreversion enables De Novo deracemization synthesis of cyclobutanes. J. Am. Chem. Soc. 146, 22840–22849 (2024).
Ci, R.-N. et al. Aliphatic C−H arylation with heteroarenes without photocatalysts. Green Chem. 25, 8500–8504 (2023).
Niu, K.-K. et al. Visible-light-mediated direct C3 alkylation of quinoxalin-2(1H)-ones using alkanes. Chem. Commun. 60, 2409–2412 (2024).
Jin, J. & MacMillan, D. W. C. Alcohols as alkylating agents in heteroarene C−H functionalization. Nature 525, 87–90 (2015).
Wang, W.-Y., Wang, J.-J., Pan, J.-B., Xiao, L.-J. & Zhou, Q.-L. Enantioselective reductive C−O bond cleavage driven by photoinduced electron transfer. Chem 10, 3667–3677 (2024).
Cao, Y., Zhang, S. & Antilla, J. C. Catalytic asymmetric 1,4-reduction of α branched 2-vinylazaarenes by a chiral SPINOL-derived borophosphate. ACS Catal. 10, 10914–10919 (2020).
Yu, Z.-X., Ma, S.-W., Li, G.-X. & Ma, L.-J. Photocatalytic carbamoyl radical transfer to alkenyl azaarenes. Synlett 35, 1883–1888 (2024).
Chelucci, G., Baldino, S., Chessa, S., Pinnab, G. A. & Soccolini, F. An easy route to optically active 1-substituted-1-pyridyl-methylamines by diastereoselective reduction of enantiopure-tert-butanesulfinyl ketimines. Tetrahedron Asymmetry 17, 3163–3169 (2006).
Kane, D. L., Figula, B. C., Balaraman, K., Bertke, J. A. & Wolf, C. General alkyl fluoride functionalization via short-lived carbocation-organozincate ion pairs. Nat. Commun. 15, 1866 (2024).
Chen, X. et al. Aerobic redox deracemization of α-aryl glycine esters. Tetrahedron Lett. 61, 152107 (2020).
Henseler, A., Kato, M., Mori, K. & Akiyama, T. Chiral phosphoric acid catalyzed transfer hydrogenation: facile synthetic access to highly optically active trifluoromethylated amines. Angew. Chem. Int. Ed. 50, 8180–8183 (2011).
Liu, Y. et al. Transition-metal free chemoselective hydroxylation and hydroxylation−deuteration of heterobenzylic methylenes. Org. Lett. 22, 8127–8131 (2020).
Acknowledgements
This work was financially supported by the National Science Foundation of China (nos. 21925103, 22301066, 22171072, 22201068 and 22301061) and Henan Normal University.
Author information
Authors and Affiliations
Contributions
Z.J. conceived of and designed the experiments. T.S., Z.L., F.N. and Q.L. performed the experiments. T.S., Z.L., F.N. and X.Z. analysed and interpreted the results. T.S. prepared the Supplementary Information. Z.J. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Jia-Rong Chen, Charnsak Thongsornkleeb and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
(1) General information; (2) general experimental procedures; (3) selected results of screening reaction conditions; (4) control experiments; (5) mechanism studies; (6) Stern–Volmer quenching studies; (7) deuterium experiments and plausible mechanism; (8) determination of absolute configurations; (9) characterization of adducts; (10) spectral data; (11) references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, T., Li, Z., Nie, F. et al. Leveraging electron donor–acceptor complexes for kinetic resolution in catalytic asymmetric photochemical synthesis. Nat. Chem. 17, 1722–1731 (2025). https://doi.org/10.1038/s41557-025-01973-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01973-y
This article is cited by
-
Enantioselective light absorption drives kinetic resolution
Nature Chemistry (2025)