Abstract
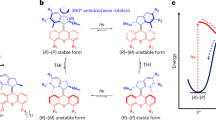
Artificial molecular motors are at the forefront of research in nanotechnology due to their ability to perform tasks by harnessing directionally controlled motion at the molecular scale. The development of light-driven nanomotors is a particularly challenging task that holds great potential for the development of sunlight-powered systems and active materials. Here we describe an azoimidazolium photochemical molecular rotary motor which operates along a triangular reaction cycle exploiting the formation of diastereomeric species upon photoisomerisation. The different thermal stability and photochemical reactivity of these diastereomers permit net directional motion combining a thermal rotation about a C–N single bond and two light-induced configurational rearrangements that proceed predominantly through a rotational mechanism, as corroborated by computational studies. The composition of the dissipative state obtained upon continuous supply of light can be modified by changing the irradiation wavelength, and as a result, the preferred rotation direction of the motor is inverted.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The online version of this Article provides Supplementary Information, including Supplementary Figs. 1–69, general methods, detailed experimental and analytical data, NMR spectra, UV–visible light spectra, computed geometries, NMR chemical shifts and ground and excited state potential energy surfaces, as well as all the additional supporting data for the study. A movie illustrating the directionally biased isomer interconversion at the ground state, experimental and mathematically extrapolated UV–visible light absorption spectra, NMR photokinetic concentration profiles, coordinates of optimized minima, transition states and minimum energy crossing points, input files for minimum energy crossing points optimizations and energies and oscillator strengths of the excited states for minima and transition states computed with multiple DFT functionals and at the CASPT2 level are provided as additional Supplementary Information.
References
Baker, M. A. B. & Berry, R. M. An introduction to the physics of the bacterial flagellar motor: a nanoscale rotary electric motor. Contemp. Phys. 50, 617–632 (2009).
Schliwa, M. Molecular Motors (Wiley, 2004).
Vale, R. D. & Milligan, R. A. The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95 (2000).
Brown, A. I. & Sivak, D. A. Theory of nonequilibrium free energy transduction by molecular machines. Chem. Rev. 120, 434–459 (2020).
Goodsell, D. S. The Machinery of Life (Copernicus, 2009).
Aprahamian, I. The future of molecular machines. ACS Cent. Sci. 6, 347–358 (2020).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Astumian, R. D. Kinetic asymmetry and directionality of nonequilibrium molecular systems. Angew. Chem. Int. Ed. 63, e202306569 (2024).
Mondal, A., Toyoda, R., Costil, R. & Feringa, B. L. Chemically driven rotatory molecular machines. Angew. Chem. Int. Ed. 61, e20220663 (2022).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Corra, S., Curcio, M., Baroncini, M., Silvi, S. & Credi, A. Photoactivated artificial molecular machines that can perform tasks. Adv. Mater. 32, 1906064 (2020).
Baroncini, M., Silvi, S. & Credi, A. Photo- and redox-driven artificial molecular motors. Chem. Rev. 120, 200–268 (2020).
Pooler, D. R. S., Lubbe, A. S., Crespi, S. & Feringa, B. L. Designing light-driven rotary molecular motors. Chem. Sci. 12, 14964–14986 (2021).
Oruganti, B., Wang, J. & Durbeej, B. Quantum chemical design of rotary molecular motors. Int. J. Quantum Chem. 118, e25405 (2018).
Sheng, J. et al. Formylation boosts the performance of light-driven overcrowded alkene-derived rotary molecular motors. Nat. Chem. 16, 1330–1338 (2024).
Boursalian, G. B. et al. All-photochemical rotation of molecular motors with a phosphorus stereoelement. J. Am. Chem. Soc. 142, 16868–16876 (2020).
Roke, D., Wezenberg, S. J. & Feringa, B. L. Molecular rotary motors: unidirectional motion around double bonds. Proc. Natl Acad. Sci. USA 115, 9423–9431 (2018).
Kistemaker, J. C. M. et al. Third-generation light-driven symmetric molecular motors. J. Am. Chem. Soc. 139, 9650–9661 (2017).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Greb, L., Eichhofer, A. & Lehn, J.-M. Synthetic molecular motors: thermal N inversion and directional photoinduced CN bond rotation of camphorquinone imines. Angew. Chem. Int. Ed. 54, 14345–14348 (2015).
Greb, L. & Lehn, J.-M. Light-driven molecular motors: imines as four-step or two-step unidirectional rotors. J. Am. Chem. Soc. 136, 13114–13117 (2014).
Gerwien, A., Gnannt, F., Mayer, P. & Dube, H. Photogearing as a concept for translation of precise motions at the nanoscale. Nat. Chem. 14, 670–676 (2022).
Wilcken, R. et al. Complete mechanism of hemithioindigo motor rotation. J. Am. Chem. Soc. 140, 5311–5318 (2018).
Gerwien, A., Mayer, P. & Dube, H. Photon-only molecular motor with reverse temperature-dependent efficiency. J. Am. Chem. Soc. 140, 16442–16445 (2018).
Guentner, M. et al. Sunlight-powered kHz rotation of a hemithioindigo-based molecular motor. Nat. Commun. 6, 8406 (2015).
Kuntze, K. et al. A visible-light-driven molecular motor based on barbituric acid. Chem. Sci. 14, 8458–8465 (2023).
Filatov, M. et al. Towards the engineering of a photon-only two-stroke rotary molecular motor. Nat. Commun. 13, 6433 (2022).
Pooler, D. R. S., Doellerer, D., Crespi, S. & Feringa, B. L. Controlling rotary motion of molecular motors based on oxindole. Org. Chem. Front. 9, 2084–2092 (2022).
Perrot, A., Wang, W., Buhler, E., Moulin, E. & Giuseppone, N. Bending actuation of hydrogels through rotation of light-driven molecular motors. Angew. Chem. Int. Ed. 62, e202300263 (2023).
Chen, J. et al. Artificial muscle-like function from hierarchical supramolecular assembly of photoresponsive molecular motors. Nat. Chem. 10, 132–138 (2018).
Foy, J. T. et al. Dual-light control of nanomachines that integrate motor and modulator subunits. Nat. Nanotechnol. 12, 540–545 (2017).
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nat. Nanotechnol. 10, 161–165 (2015).
Qutbuddin, Y. et al. Light-activated synthetic rotary motors in lipid membranes induce shape changes through membrane expansion. Adv. Mater. 36, 2311176 (2024).
Li, Q., Tan, J. & Sun, T. Light-driven Feringa motors for precision molecular mechanotherapeutics. Trends Chem. 5, 653–656 (2023).
Guinart, A. et al. Synthetic molecular motor activates drug delivery from polymersomes. Proc. Natl Acad. Sci. USA 120, e2301279120 (2023).
Corra, S., Curcio, M. & Credi, A. Photoactivated artificial molecular motors. JACS Au 3, 1301–1313 (2023).
Zhang, Q., Qu, D.-H., Tian, H. & Feringa, B. L. Bottom-up: can supramolecular tools deliver responsiveness from molecular motors to macroscopic materials? Matter 3, 355–370 (2020).
Jerca, F. A., Jerca, V. V. & Hoogenboom, R. Advances and opportunities in the exciting world of azobenzenes. Nat. Rev. Chem. 6, 51–69 (2022).
Asaka, T., Akai, N., Kawai, A. & Shibuya, K. Photochromism of 3-butyl-1-methyl-2-phenylazoimidazolium in room temperature ionic liquids. J. Photochem. Photobiol. A 209, 12–18 (2010).
Borsley, S., Kreidt, E., Leigh, D. A. & Roberts, B. M. W. Autonomous fuelled directional rotation about a covalent single bond. Nature 604, 80–85 (2022).
Greenfield, J. L. et al. Molecular Photoswitches (ed. Pianowski, Z. L.) Ch. 5 (Wiley, 2022).
Lin, I. J. B. & Vasam, C. S. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 251, 642–670 (2007).
Nicoli, F. et al. Photoinduced autonomous nonequilibrium operation of a molecular shuttle by combined isomerization and proton transfer through a catalytic pathway. J. Am. Chem. Soc. 144, 10180–10185 (2022).
Hugelshofer, C. L., Mellem, K. T. & Myers, A. G. Synthesis of quaternary α-methyl α-amino acids by asymmetric alkylation of pseudoephenamine alaninamide pivaldimine. Org. Lett. 15, 3134–3137 (2013).
Onsager, L. Reciprocal relations in irreversible processes. Phys. Rev. 37, 405–426 (1931).
Astumian, R. D. Microscopic reversibility as the organizing principle of molecular machines. Nat. Nanotechnol. 7, 684–688 (2012).
Uhl, E., Thumser, S., Mayer, P. & Dube, H. Transmission of unidirectional molecular motor rotation to a remote biaryl axis. Angew. Chem. Int. Ed. 57, 11064–11068 (2018).
Weingart, O., Lan, Z., Thiel, W. & Thiel, W. Chiral pathways and periodic decay in cis-azobenzene photodynamics. J. Phys. Chem. Lett. 2, 1506–1509 (2011).
Wang, Y.-T. et al. Photoisomerization of arylazopyrazole photoswitches: stereospecific excited-state relaxation. Angew. Chem. Int. Ed. 55, 14009–14013 (2016).
Aleotti, F. et al. Multidimensional potential energy surfaces resolved at the RASPT2 level for accurate photoinduced isomerization dynamics of azobenzene. J. Chem. Theory Comput. 15, 6813–6823 (2019).
Nenov, A. et al. UV-light-induced vibrational coherences: the key to understand Kasha rule violation in trans-azobenzene. J. Phys. Chem. Lett. 9, 1534–1541 (2018).
Casellas, J., Bearpark, M. J. & Reguero, M. Excited-state decay in the photoisomerization of azobenzene: a new balance between mechanisms. ChemPhysChem 17, 3068–3079 (2016).
Moghaddam, K. G., Giudetti, G., Sipma, W. & Faraji, S. Theoretical insights into the effect of size and substitution patterns of azobenzene derivatives on the DNA G-quadruplex. Phys. Chem. Chem. Phys. 22, 26944–26954 (2020).
Vela, S., Krüger, C. & Corminboeuf, C. Exploring chemical space in the search for improved azoheteroarene-based photoswitches. Phys. Chem. Chem. Phys. 21, 20782–20790 (2019).
Corra, S. et al. Kinetic and energetic insights into the dissipative non-equilibrium operation of an autonomous light-powered supramolecular pump. Nat. Nanotechnol. 17, 746–751 (2022).
Sangchai, T., Al Shehimy, S., Penocchio, E. & Ragazzon, G. Artificial molecular ratchets: tools enabling endergonic processes. Angew. Chem. Int. Ed. 62, e202309501 (2023).
Falivene, L. et al. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 11, 872–879 (2019).
Acknowledgements
This work was financially supported by the Royal Society of Chemistry (M.C., Research Fund R23-8129362510), the European Union—Next Generation EU and the Italian Ministry of University and Research (A.C., PRIN grant no. 2022JMTLE; M.B. and C.T., PRIN grant no. 2022KMMAYM_002; S.S., PRIN grant no. 201732PY3X; M.G. and F.A., PRIN grant no. P20224AWLB) and the University of Bologna. Correspondence should be addressed to M.C.
Author information
Authors and Affiliations
Contributions
M.C. and F.N. conceived the project. M.C., M.B., S.S. and L.M. guided and supervised the research. F.N., S.B. and M.C. synthesized the compounds and carried out NMR experiments. C.T. carried out the photophysical characterisations. E.L. and F.A. conducted computations. A.C., M.B., M.C., S.S. and M.G. secured research funds. M.C., C.T., F.N., E.L. and L.M. wrote the paper. All authors discussed the results and commented the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Stefano Crespi, Jin Wen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, synthetic procedures (Supplementary Fig. 1), NMR data (Supplementary Figs. 2–32), photophysical data (Supplementary Figs. 33–51 and Supplementary Table 1), computational details, computational data (Supplementary Figs. 52–68 and Supplementary Tables 2–13) and kinetic analysis (Supplementary Fig. 69).
Supplementary Video 1
Movie illustrating the directional isomer interconversion at the ground state.
Supplementary Data 2
Absorption spectra of the E and Z isomers of the three reported compounds.
Supplementary Data 3
Photokinetic traces of the light-on experiments upon irradiation at 365 and 453 nm (compare with Fig. 5).
Supplementary Data 4
Coordinates (xyz) of all optimized minima, transition states (TS) and minimum energy crossing points (MECP), along with example Gaussian input files for DFT calculations in chloroform.
Supplementary Data 5
Input files for minimum energy crossing points (MECP) optimizations performed with COBRAMM at the CASPT2 level in the gas phase and in chloroform.
Supplementary Data 6
Energies and oscillator strengths of the excited states for each minimum (E, ZA and ZB) and transition state (ZA–ZB) computed with multiple DFT functionals and at the CASPT2 level in gas phase and in chloroform.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nicoli, F., Taticchi, C., Lorini, E. et al. Wavelength-steered directional rotation in an autonomous light-driven molecular motor. Nat. Chem. (2026). https://doi.org/10.1038/s41557-025-02045-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41557-025-02045-x