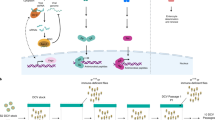
Abstract
Immune systems are of pivotal importance to any living organism on Earth, as they protect the organism against deleterious effects of viral infections. Though the current knowledge about these systems is still biased towards the immune response in vertebrates, some studies have focused on the identification and characterization of components of invertebrate antiviral immune systems. Two classic model organisms, the insect Drosophila melanogaster and the nematode Caenorhabditis elegans, were instrumental in the discovery of several important components of the innate immune system, such as the Toll-like receptors and the RNA interference pathway. However, these two model organisms provide only a limited view of the evolutionary history of the immune system, as they both are ecdysozoan protostomes. Recent functional studies in non-classic models such as unicellular holozoans (for example, choanoflagellates), lophotrochozoans (for example, oysters) and cnidarians (for example, sea anemones) have added crucial information for understanding the evolution of antiviral systems, as they revealed unexpected ancestral complexity. This Review aims to summarize this information and present the ancestral nature of the antiviral immune response in animals. We also discuss lineage-specific adaptations and future perspectives for the comparative study of the innate immune system that are essential for understanding its evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Harvey, E. & Holmes, E. C. Diversity and evolution of the animal virome. Nat. Rev. Microbiol. 20, 321–334 (2022).
Flajnik, M. F. & du Pasquier, L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 25, 640–644 (2004).
Negishi, H., Taniguchi, T. & Yanai, H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb. Perspect. Biol 10, a028423 (2018).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–793 (2009).
Zhou, A., Hassel, B. A. & Silverman, R. H. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell 72, 753–765 (1993).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Maillard, P. V., Veen, A. G., Poirier, E. Z. & Reis e Sousa, C. Slicing and dicing viruses: antiviral RNA interference in mammals. EMBO J. 38, e100941 (2019).
Wang, W., Xu, L., Su, J., Peppelenbosch, M. P. & Pan, Q. Transcriptional regulation of antiviral interferon-stimulated genes. Trends Microbiol. 25, 573–584 (2017).
Stirnweiss, A. et al. IFN Regulatory factor-1 bypasses IFN-mediated antiviral effects through Viperin gene induction. J. Immun. 184, 5179–5185 (2010).
Tamura, T., Yanai, H., Negishi, H. & Taniguchi, T. The IRF family of transcription factors: inception, impact and implications in oncogenesis. OncoImmunology 1, 1376–1386 (2012).
Taniguchi, K. & Karin, M. NF-kB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Andreeva, L. et al. CGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 549, 394–398 (2017).
Maekawa, H. et al. Mitochondrial damage causes inflammation via cGAS–STING signaling in acute kidney injury. Cell Rep. 29, 1261–1273.e6 (2019).
Chen, H. et al. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell 147, 436–446 (2011).
Abe, T. & Barber, G. N. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J. Virol. 88, 5328–5341 (2014).
McWhirter, S. M. et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med. 206, 1899–1911 (2009).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Cui, Y. et al. The Stat3/5 locus encodes novel endoplasmic reticulum and helicase-like proteins that are preferentially expressed in normal and neoplastic mammary tissue. Genomics 78, 129–134 (2001).
Schimidt, A. et al. 5-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl Acad. Sci. USA 106, 12067–12072 (2009).
Schlee, M. et al. Recognition of 5′-triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Hornung, V. et al. 5′-triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Pichlmair, A. et al. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 83, 10761–10769 (2009).
Wu, B. et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152, 276–289 (2013).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 (2005).
Goubau, D., Deddouche, S. & Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 38, 855–869 (2013).
Bilak, H., Tauszig-Delamasure, S. & Imler, J. L. Toll and Toll-like receptors in Drosophila. Biochem. Soc. Trans. 31, 648–651 (2003).
Anthoney, N., Foldi, I. & Hidalgo, A. Toll and Toll-like receptor signalling in development. Development 145, dev156018 (2018).
Fitzgerald, K. A. & Kagan, J. C. Toll-like receptors and the control of immunity. Cell 180, 1044–1066 (2020).
Orús-Alcalde, A., Lu, T. M., Børve, A. & Hejnol, A. The evolution of the metazoan Toll receptor family and its expression during protostome development. BMC Ecol. Evol. 21, 208 (2021).
Leulier, F. & Lemaitre, B. Toll-like receptors—taking an evolutionary approach. Nat. Rev. Genet. 9, 165–178 (2008).
Wilkins, C. et al. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 436, 1044–1047 (2005).
Wang, X. H. et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312, 452–454 (2006).
Keene, K. M. et al. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Natl Acad. Sci. USA 101, 17240–17245 (2004).
Hamilton, A. J. & Baulcombe, D. C. A species of small antisense RNA in post-transcriptional gene silencing in plants. Science 286, 950–952 (1999).
Papaefthimiou, I. et al. Replicating potato spindle tuber viroid RNA is accompanied by short RNA fragments that are characteristic of post-transcriptional gene silencing. Nucleic Acids Res. 29, 2395–2400 (2001).
Hutvágner, G. et al. A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temperal RNA. Science 293, 834–838 (2001).
Chendrimada, T. P. et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744 (2005).
Hammond, S. M., Bernstein, E., Beach, D. & Hannon, G. J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296 (2000).
Bernstein, R., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Elbashir, S. M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001).
Parker, J. S., Roe, S. M. & Barford, D. Structural insights into mRNA recognition from a PIWI domain–siRNA guide complex. Nature 434, 663–666 (2005).
Cenik, E. S. & Zamore, P. D. Argonaute proteins. Curr. Biol. 21, 446–449 (2011).
Berkhout, B. RNAi-mediated antiviral immunity in mammals. Curr. Opin. Virol. 32, 9–14 (2018).
Pfeffer, S. et al. Identification of virus-encoded microRNAs. Crit. Rev. Biochem. Mol. Biol. 304, 734–736 (2001).
Kennedy, E. M. et al. Production of functional small interfering RNAs by an amino-terminal deletion mutant of human Dicer. Proc. Natl Acad. Sci. USA 112, E6945–E6954 (2015).
Weng, K. F. et al. A cytoplasmic RNA virus generates functional viral small RNAs and regulates viral IRES activity in mammalian cells. Nucleic Acids Res. 42, 12789–12805 (2014).
Parameswaran, P. et al. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog. 6, e1000764 (2010).
Chen, G. R., Sive, H. & Bartel, D. P. A Seed mismatch enhances Argonaute2-catalyzed cleavage and partially rescues severely impaired cleavage found in fish. Mol. Cell 68, 1095–1107.e5 (2017).
Flemr, M. et al. XA retrotransposon-driven dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell 155, 807–816 (2013).
Cullen, B. R. Is RNA interference involved in intrinsic antiviral immunity in mammals? Nat. Immunol. 7, 563–567 (2006).
Watson, S. F., Knol, L. I., Witteveldt, J. & Macias, S. Crosstalk between mammalian antiviral pathways. Non-coding RNA 5, 29 (2019).
Poirier, E. Z. et al. An isoform of Dicer protects mammalian stem cells against multiple RNA viruses. Science 373, 231–236 (2021).
Laumer, C. E. et al. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. R. Soc. B 286, 20190831 (2019).
Dostert, C. et al. The Jak–STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 6, 946–953 (2005).
Paradkar, P. N., Trinidad, L., Voysey, R., Duchemin, J. B. & Walker, P. J. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak–STAT pathway. Proc. Natl Acad. Sci. USA 109, 18915–18920 (2012).
Li, C. et al. Activation of Vago by interferon regulatory factor (IRF) suggests an interferon system-like antiviral mechanism in shrimp. Sci. Rep. 5, 15078 (2015).
Nehyba, J., Hrdličková, R. & Bose, H. R. in Encyclopedia of Life Sciences (eLS), https://doi.org/10.1002/9780470015902.a0022874 (2010).
Nehyba, J., Hrdličková, R. & Bose, H. R. Dynamic evolution of immune system regulators: the history of the interferon regulatory factor family. Mol. Biol. Evol. 26, 2539–2550 (2009).
Deddouche, S. et al. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in Drosophila. Nat. Immunol. 9, 1425–1432 (2008).
Holleufer, A. et al. Two cGAS-like receptors induce antiviral immunity in Drosophila. Nature 597, 114–118 (2021).
Slavik, K. M. et al. cGAS-like receptors sense RNA and control 3′2′-cGAMP signalling in Drosophila. Nature 597, 109–113 (2021).
Cai, H. et al. 2′3′-cGAMP triggers a STING- and NF-κB-dependent broad antiviral response in Drosophila. Sci. Signal. 13, eabc4537 (2020).
Goto, A. et al. The kinase IKKβ regulates a STING- and NF-κB-dependent antiviral response pathway in Drosophila. Immunity 49, 225–234.e4 (2018).
Segrist, E., Dittmar, M., Gold, B. & Cherry, S. Orally acquired cyclic dinucleotides drive dSTING-dependent antiviral immunity in enterocytes. Cell Rep. 37, 110150 (2021).
Martin, M., Hiroyasu, A., Guzman, R. M., Roberts, S. A. & Goodman, A. G. Analysis of Drosophila STING reveals an evolutionarily conserved antimicrobial function. Cell Rep. 23, 3537–3550 (2018).
Liu, Y. et al. Inflammation-induced, STING-dependent autophagy restricts Zika virus infection in the Drosophila brain. Cell Host Microbe 24, 57–68 (2018).
Levashina, E. A. et al. Constitutive activation of Toll-mediated antifungal defense in Serpin-deficient Drosophila. Science 285, 1917–1919 (1999).
Ooi, J. Y., Yagi, Y., Hu, X. & Ip, Y. T. The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 3, 82–87 (2002).
Tauszig, S., Jouanguy, E., Hoffmann, J. A. & Imler, J. L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl Acad. Sci. USA 97, 10520–10525 (2000).
Orús-Alcalde, A., Børve, A. & Hejnol, A. The Toll and Imd pathway, the complement system and lectins during immune response of the nemertean Lineus ruber. Preprint at https://doi.org/10.1101/2022.04.26.489627 (2022).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Félix, M. A. et al. Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biol. 9, e1000586 (2011).
Ashe, A. et al. A deletion polymorphism in the Caenorhabditis elegans RIG-I homolog disables viral RNA dicing and antiviral immunity. eLife 2, e00994 (2013).
Tanguy, M. et al. An alternative STAT signaling pathway acts in viral immunity in Caenorhabditis elegans. mBio 8, e00927-17 (2017).
Duchaine, T. F. et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell 124, 343–354 (2006).
Coffman, S. R. et al. Caenorhabditis elegans RIG-I homolog mediates antiviral RNA interference downstream of Dicer-dependent biogenesis of viral small interfering RNAs. mBio 8, e00264-17 (2017).
Guo, X., Zhang, R., Wang, J., Ding, S. W. & Lu, R. Homologous RIG-I-like helicase proteins direct RNA-mediated antiviral immunity in C. elegans by distinct mechanisms. Proc. Natl Acad. Sci. USA 110, 16085–16090 (2013).
Li, K. et al. Insights into the structure and RNA-binding specificity of Caenorhabditis elegans Dicer-related helicase 3 (DRH-3). Nucleic Acids Res. 49, 9978–9991 (2021).
Palmer, W. H., Hadfield, J. D. & Obbard, D. J. RNA-interference pathways display high rates of adaptive protein evolution in multiple invertebrates. Genetics 208, 1585–1599 (2018).
Lewis, S. H. et al. Pan-arthropod analysis reveals somatic piRNAs as an ancestral defence against transposable elements. Nat. Ecol. Evol. 2, 174–181 (2018).
Wein, T. & Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. 22, 629–638 (2022).
Huang, B. et al. Characterization of the mollusc RIG-I/MAVS pathway reveals an archaic antiviral signalling framework in invertebrates. Sci. Rep. 7, 8217 (2017).
de Jong, D. et al. Multiple Dicer genes in the early-diverging Metazoa. Mol. Biol. Evol. 26, 1333–1340 (2009).
Dominguez-Huerta, G. et al. Diversity and ecological footprint of global ocean RNA viruses. Science 376, 1202–1208 (2022).
Zayed, A. A. et al. Cryptic and abundant marine viruses at the evolutionary origins of Earth’s RNA virome. Science 376, 156–162 (2022).
Callanan, J. et al. Expansion of known ssRNA phage genomes: from tens to over a thousand. Sci. Adv. 6, eaay5981 (2020).
Steward, G. F. et al. Are we missing half of the viruses in the ocean? ISME J. 7, 672–679 (2013).
Woznica, A. et al. STING mediates immune responses in the closest living relatives of animals. eLife 10, e70436 (2021).
Margolis, S. R. & Dietzen, P. A. The cyclic dinucleotide 2′3′-cGAMP induces a broad antibacterial and antiviral response in the sea anemone Nematostella vectensis. Proc. Natl Acad. Sci. USA 118, e2109022118 (2021).
Whiteley, A. T. et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567, 194–199 (2019).
Cohen, D. et al. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature 574, 691–695 (2019).
Morehouse, B. R. et al. STING cyclic dinucleotide sensing originated in bacteria. Nature 586, 429–433 (2020).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018).
Bernheim, A. et al. Prokaryotic viperins produce diverse antiviral molecules. Nature 589, 120–124 (2021).
Makarova, K. S., Wolf, Y. I., van der Oost, J. & Koonin, E. V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol. Direct 4, 29 (2009).
Brennan, J. J. et al. Sea anemone model has a single Toll-like receptor that can function in pathogen detection, NF-κB signal transduction, and development. Proc. Natl Acad. Sci. USA 114, E10122–E10131 (2017).
Poole, A. Z. & Weis, V. M. TIR-domain-containing protein repertoire of nine anthozoan species reveals coral-specific expansions and uncharacterized proteins. Dev. Comp. Immunol. 46, 480–488 (2014).
Williams, L. M. et al. A conserved Toll-like receptor-to-NF-κB signaling pathway in the endangered coral Orbicella faveolata. Dev. Comp. Immunol. 79, 128–136 (2018).
Brennan, J. J. & Gilmore, T. D. Evolutionary origins of Toll-like receptor signalling. Mol. Biol. Evol. 35, 1576–1587 (2018).
Roesel, C. L. & Vollmer, S. V. Differential gene expression analysis of symbiotic and aposymbiotic Exaiptasia anemones under immune challenge with Vibrio coralliilyticus. Ecol. Evol. 9, 8279–8293 (2019).
Davidson, C. R., Best, N. M., Francis, J. W., Cooper, E. L. & Wood, T. C. Toll-like receptor genes (TLRs) from Capitella capitata and Helobdella robusta (Annelida). Dev. Comp. Immunol. 32, 608–612 (2008).
Tassia, M. G., Whelan, N. V. & Halanych, K. M. Toll-like receptor pathway evolution in deuterostomes. Proc. Natl Acad. Sci. USA 114, 7055–7060 (2017).
Ji, J. et al. Characterization of the TLR family in Branchiostoma lanceolatum and discovery of a novel TLR22-like involved in dsRNA recognition in amphioxus. Front Immunol. 9, 2525 (2018).
Hibino, T. et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 300, 349–365 (2006).
Zhang, Y. et al. Characteristic and functional analysis of Toll-like receptors (TLRs) in the lophotrocozoan, Crassostrea gigas, reveals ancient origin of TLR-mediated innate immunity. PLoS ONE 8, e76464 (2013).
Sarkar, D., Desalle, R. & Fisher, P. B. Evolution of MDA-5/RIG-I-dependent innate immunity: independent evolution by domain grafting. Proc. Natl Acad. Sci. USA 105, 17040–17045 (2008).
Zou, J., Chang, M., Nie, P. & Secombes, C. J. Origin and evolution of the RIG-I like RNA helicase gene family. BMC Evol. Biol. 9, 85 (2009).
Mukherjee, K., Korithoski, B. & Kolaczkowski, B. Ancient origins of vertebrate-specific innate antiviral immunity. Mol. Biol. Evol. 31, 140–153 (2014).
Zhang, L. et al. Massive expansion and functional divergence of innate immune genes in a protostome. Sci. Rep. 5, 8693 (2015).
Lewandowska, M., Sharoni, T., Admoni, Y., Aharoni, R. & Moran, Y. Functional characterization of the cnidarian antiviral immune response reveals ancestral complexity. Mol. Biol. Evol. 38, 4546–4561 (2021).
Xu, L. et al. Loss of RIG-I leads to a functional replacement with MDA5 in the Chinese tree shrew. Proc. Natl Acad. Sci. USA 113, 10950–10955 (2016).
Krchlíková, V. et al. Repeated MDA5 gene loss in birds: an evolutionary perspective. Viruses 13, 2131 (2021).
Fischer, H., Tschachler, E. & Eckhart, L. Pangolins lack IFIH1/MDA5, a cytoplasmic RNA sensor that initiates innate immune defense upon coronavirus infection. Front. Immunol. 11, 939 (2020).
Huang, B. et al. The first identified invertebrate LGP2-like homolog gene in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 128, 238–245 (2022).
Lindbo, J. A., Silva-Rosales, L., Proebsting, W. M. & Dougherty, W. G. Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance. Plant Cell 5, 1749–1759 (1993).
Torres-Martínez, S. & Ruiz-Vázquez, R. M. The RNAi universe in fungi: a varied landscape of small RNAs and biological functions. Annu. Rev. Microbiol. 71, 371–391 (2017).
Bonning, B. C. & Saleh, M. C. The interplay between viruses and RNAi pathways in insects. Annu. Rev. Entomol. 66, 61–79 (2021).
Kuzmenko, A. et al. DNA targeting and interference by a bacterial Argonaute nuclease. Nature 587, 632–637 (2020).
Olovnikov, I., Chan, K., Sachidanandam, R., Newman, D. K. & Aravin, A. A. Bacterial Argonaute samples the transcriptome to identify foreign DNA. Mol. Cell. 51, 594–605 (2013).
Swarts, D. C. et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature 507, 258–261 (2014).
Mukherjee, K., Campos, H. & Kolaczkowski, B. Evolution of animal and plant dicers: early parallel duplications and recurrent adaptation of antiviral RNA binding in plants. Mol. Biol. Evol. 30, 627–641 (2013).
Lee, Y. S. et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81 (2004).
Galiana-Arnoux, D., Dostert, C., Schneemann, A., Hoffmann, J. A. & Imler, J. L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 7, 590–597 (2006).
Moran, Y., Praher, D., Fredman, D. & Technau, U. The evolution of microRNA pathway protein components in Cnidaria. Mol. Biol. Evol. 30, 2541–2552 (2013).
Veen, A. G. et al. The RIG‐I‐like receptor LGP2 inhibits Dicer‐dependent processing of long double‐stranded RNA and blocks RNA interference in mammalian cells. EMBO J. 37, e97479 (2018).
Witteveldt, J., Ivens, A. & Macias, S. Inhibition of microprocessor function during the activation of the type I Interferon response. Cell Rep. 23, 3275–3285 (2018).
Waldron, F. M., Stone, G. N. & Obbard, D. J. Metagenomic sequencing suggests a diversity of RNA interference-like responses to viruses across multicellular eukaryotes. PLoS Genet. 14, e1007533 (2018).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6, 644–658 (2006).
Leger, M. M., Ros-Rocher, N., Najle, S. R. & Ruiz-Trillo, I. Rel/NF-κB transcription factors emerged at the onset of opisthokonts. Genome Biol. Evol. 14, evab289 (2022).
Mercurio, F. & Manning, A. M. NF-kB as a primary regulator of the stress response. Oncogene 18, 6163–6171 (1999).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 20, 89–108 (2019).
Sarkar, A., Volff, J. N. & Vaury, C. piRNAs and their diverse roles: a transposable element-driven tactic for gene regulation? FASEB J. 31, 436–446 (2017).
Aravin, A. A. et al. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11, 1017–1027 (2001).
Miesen, P., Girardi, E. & van Rij, R. P. Distinct sets of PIWI proteins produce arbovirus and transposon-derived piRNAs in Aedes aegypti mosquito cells. Nucleic Acids Res. 43, 6545–6556 (2015).
Morazzani, E. M., Wiley, M. R., Murreddu, M. G., Adelman, Z. N. & Myles, K. M. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 8, e1002470 (2012).
Miesen, P., Joosten, J. & van Rij, R. P. PIWIs go viral: arbovirus-derived piRNAs in vector mosquitoes. PLoS Pathog. 12, e1006017 (2016).
Joosten, J., Overheul, G. J., van Rij, R. P. & Miesen, P. Endogenous piRNA-guided slicing triggers responder and trailer piRNA production from viral RNA in Aedes aegypti mosquitoes. Nucleic Acids Res. 49, 8886–8899 (2021).
Petit, M. et al. piRNA pathway is not required for antiviral defense in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 113, E4218–E4227 (2016).
Tassetto, M., Kunitomi, M. & Andino, R. Circulating immune cells mediate a systemic RNAi-based adaptive antiviral response in Drosophila. Cell 169, 314–325 (2017).
Silverman, N. et al. Control of RNA viruses in mosquito cells through the acquisition of vDNA and endogenous viral elements. eLife 8, e41244 (2019).
Chan, Y. K. & Gack, M. U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 14, 360–373 (2016).
Christensen, M. H. & Paludan, S. R. Viral evasion of DNA-stimulated innate immune responses. Cell Mol. Immunol. 14, 4–13 (2017).
Koonin, E. V. & Dolja, V. V. A virocentric perspective on the evolution of life. Curr. Opin. Virol. 3, 546–557 (2013).
Drinnenberg, I. A. et al. Compatibility with killer explains the rise of RNAi-deficient fungi. Science 333, 1592 (2011).
Nicolás, F. E. & Garre, V. RNA interference in Fungi: retention and loss. Microbiol. Spectr. 4, 657–671 (2016).
Nicolás, F. E., Torres-Martínez, S. & Ruiz-Vázquez, R. M. Loss and retention of RNA interference in Fungi and parasites. PLoS Pathog. 9, e1003089 (2013).
Mongelli, V. et al. Innate immune pathways act synergistically to constrain RNA virus evolution in Drosophila melanogaster. Nat. Ecol. Evol. 6, 565–578 (2022).
Phillips, J. E., Santos, M., Konchwala, M., Xing, C. & Pan, D. Genome editing in the unicellular holozoan Capsaspora owczarzaki suggests a premetazoan role for the Hippo pathway in multicellular morphogenesis. eLife 11, e77598 (2022).
Presnell, J. S., Bubel, M., Knowles, T., Patry, W. & Browne, W. E. Multigenerational laboratory culture of pelagic ctenophores and CRISPR–Cas9 genome editing in the lobate Mnemiopsis leidyi. Nat. Protoc. 17, 1868–1900 (2022).
Chen, S. N., Zou, P. F. & Nie, P. Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) in fish: current knowledge and future perspectives. Immunology 151, 16–25 (2017).
Sabbah, A. et al. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10, 1073–1080 (2009).
Bauernfried, S., Scherr, M. J., Pichlmair, A., Duderstadt, K. E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 371, eabd0811 (2021).
Richter, D. J., Fozouni, P., Eisen, M. B. & King, N. Gene family innovation, conservation and loss on the animal stem lineage. eLife 7, e34226 (2018).
Acknowledgements
We thank S. Moineau and A. Culley (Université Laval) for advice on the diversity of DNA and RNA phages.
Author information
Authors and Affiliations
Contributions
R.E.I. and Y.M. contributed to all aspects of writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Andreas Hejnol and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwama, R.E., Moran, Y. Origins and diversification of animal innate immune responses against viral infections. Nat Ecol Evol 7, 182–193 (2023). https://doi.org/10.1038/s41559-022-01951-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-022-01951-4
This article is cited by
-
Absence of conserved immune signalling pathways and increased pathogen susceptibility associated to photosymbiosis in acoels
BMC Biology (2026)
-
Volatile and non-volatile pathogen cues shape host extracellular vesicles production in pre-infection response
Nature Communications (2025)
-
Conservation of molecular responses upon viral infection in the non-vascular plant Marchantia polymorpha
Nature Communications (2024)
-
Viperin immunity evolved across the tree of life through serial innovations on a conserved scaffold
Nature Ecology & Evolution (2024)
-
Adaptation in human immune cells residing in tissues at the frontline of infections
Nature Communications (2024)