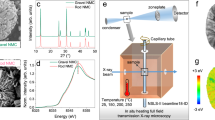
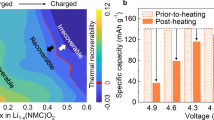
Abstract
High-nickel oxide cathodes, LiNixM1−xO2 (x ≥ 0.8), are preferred in automotive lithium batteries, but they face thermal instability challenges. Inconsistent literature reports and unstandardized testing protocols further complicate quantitative assessments of the thermal stability of these cathodes. We present here a statistical thermal analysis based on the differential scanning calorimetry measurements of 15 representative cathode materials with different compositions, morphologies and states of charge. The findings reveal that each cathode has a critical state of charge that defines its safe operating limit, which is affected by the metal–oxygen bond strength and surface reactivity. The thermal runaway temperature is dictated by the layered Li1−xNiO2 to LiNi2O4 spinel-like phase transition, which is thermodynamically determined by the metal–oxygen bond covalency and kinetically influenced by the cation mixing and particle size. Raman spectroscopy is used to predict the thermal runaway temperature on the basis of the linear relationship between them. Finally, we propose a thermal stability index to quantify cathode thermal stability as a guide for developing safer high-nickel cathodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information.
Change history
28 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41560-025-01763-3
References
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Bianchini, M., Roca‐Ayats, M., Hartmann, P., Brezesinski, T. & Janek, J. There and back again—the journey of LiNiO2 as a cathode active material. Angew. Chem. 58, 10434–10458 (2019).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Kwade, A. et al. Current status and challenges for automotive battery production technologies. Nat. Energy 3, 290–300 (2018).
Brand, M. et al. Electrical safety of commercial Li-ion cells based on NMC and NCA technology compared to LFP technology. World Electr. Veh. J. 6, 572–580 (2013).
Jiang, J. & Dahn, J. R. ARC studies of the thermal stability of three different cathode materials: LiCoO2; Li[Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC electrolytes. Electrochem. Commun. 6, 39–43 (2004).
Tang, X. et al. Investigating the critical characteristics of thermal runaway process for LiFePO4/graphite batteries by a ceased segmented method. iScience 24, 103088 (2021).
Feng, X., Ren, D., He, X. & Ouyang, M. Mitigating thermal runaway of lithium-ion batteries. Joule 4, 743–770 (2020).
Feng, X. et al. Thermal runaway mechanism of lithium ion-battery for electric vehicles: a review. Energy Storage Mater. 10, 246–267 (2018).
Wang, Y. et al. Challenges and opportunities to mitigate the catastrophic thermal runaway of high‐energy batteries. Adv. Energy Mater. 13, 2203841 (2023).
Finegan, D. P. et al. Characterising thermal runaway within lithium-ion cells by inducing and monitoring internal short circuits. Energy Environ. Sci. 10, 1377–1388 (2017).
Liu, X., Stoliarov, S. I., Denlinger, M., Masias, A. & Snyder, K. Comprehensive calorimetry of the thermally induced failure of a lithium-ion battery. J. Power Sources 280, 516–525 (2015).
Liu, X. et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2, 2047–2064 (2018).
Brand, M. J. et al. Effects of vibrations and shocks on lithium-ion cells. J. Power Sources 288, 62–69 (2015).
Manikandan, B., Yap, C. & Balaya, P. Towards understanding heat generation characteristics of Li-ion batteries by calorimetry, impedance, and potentiometry studies. J. Electrochem. Soc. 164, A2794–A2800 (2017).
Feng, X., Lu, L., Ouyang, M., Li, J. & He, X. A 3D thermal runaway propagation model for a large format lithium-ion-battery module. Energy 115, 194–208 (2016).
Zheng, J. et al. Tuning of thermal stability in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 138, 13326–13334 (2016).
Bak, S.-M. et al. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials. Chem. Mater. 25, 337–351 (2013).
Nam, K.-W. et al. Combining in situ synchrotron X‐ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium‐ion batteries. Adv. Funct. Mater. 23, 1047–1063 (2013).
Ma, L., Nie, M., Xia, J. & Dahn, J. R. A systematic study on the reactivity of different grades of charged Li[NixMnyCoz]O2 with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Power Sources 327, 145–150 (2016).
Noh, H.-J., Youn, S., Yoon, C. S. & Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 233, 121–130 (2013).
Geng, L. et al. Probing thermal stability of Li-ion battery Ni-rich layered oxide cathodes by means of operando gas analysis and neutron diffraction. ACS Appl. Energy Mater. 3, 7058–7065 (2020).
Bak, S.-M. et al. Structural changes and thermal stability of Charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 6, 22594–22601 (2014).
Yoon, W.-S. et al. Time-resolved XRD study on the thermal decomposition of nickel-based layered cathode materials for Li-ion batteries. J. Power Sources 163, 219–222 (2006).
Downie, L. E. & Dahn, J. R. Determination of the voltage dependence of parasitic heat flow in lithium-ion cells using isothermal microcalorimetry. J. Electrochem. Soc. 161, A1782–A1787 (2014).
Xiong, D. J. et al. Measuring oxygen release from delithiated LiNixMnyCo1−x−yO2 and its effects on the performance of high voltage Li-ion cells. J. Electrochem. Soc. 164, A3025–A3037 (2017).
Li, H. et al. Is cobalt needed in Ni-rich positive electrode materials for lithium-ion batteries?. J. Electrochem. Soc. 166, A429–A439 (2019).
Kasnatscheew, J., Röser, S., Börner, M. & Winter, M. Do increased Ni contents in LiNixMnyCozO2 (NMC) electrodes decrease structural and thermal stability of Li-ion batteries? A thorough look by consideration of the Li+ extraction ratio. Energy Mater. 2, 7733–7737 (2019).
Matsumoto, K., Inoue, K. & Utsugi, K. A highly safe battery with a non-flammable triethyl-phosphate-based electrolyte. J. Power Sources 273, 954–958 (2015).
Huang, Z. et al. A solvent-anchored non-flammable electrolyte. Matter 6, 445–459 (2023).
Jo, E. et al. Different thermal degradation mechanisms: role of aluminum in Ni-rich layered cathode materials. Nano Energy 78, 105367 (2020).
Li, F. et al. Gradient boracic polyanion doping-derived surface lattice modulation of high-voltage Ni-rich layered cathodes for high-energy-density Li-ion batteries. ACS Energy Lett. 8, 4903–4914 (2023).
Zeng, Z. et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 3, 674–681 (2018).
Zhu, G.-R. et al. Non-flammable solvent-free liquid polymer electrolyte for lithium metal batteries. Nat. Commun. 14, 4617 (2023).
Weigel, T. et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 4, 508–516 (2019).
Gomez-Martin, A. et al. Magnesium substitution in Ni-rich NMC layered cathodes for high-energy lithium-ion batteries. Adv. Energy Mater. 12, 2103045 (2022).
Xie, Q., Li, W. & Manthiram, A. A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries. Chem. Mater. 31, 938–946 (2019).
Amalraj, S. F. et al. Boron doped Ni-rich LiNi0.85Co0.10Mn0.05O2 cathode materials studied by structural analysis, solid state NMR, computational modeling, and electrochemical performance. Energy Storage Mater. 42, 594–607 (2021).
Xie, Q., Li, W., Dolocan, A. & Manthiram, A. Insights into boron-based polyanion-tuned high-nickel cathodes for high-energy-density lithium-ion batteries. Chem. Mater. 31, 8886–8897 (2019).
Kadkhodayan, A. & Brenner, A. Temperature-programmed reduction and oxidation of metals supported on γ-alumina. J. Catal. 117, 311–321 (1989).
Li, Y. et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials. Nano Energy 85, 105878 (2021).
Hou, J. et al. Unlocking the self-supported thermal runaway of high-energy lithium-ion batteries. Energy Storage Mater. 39, 395–402 (2021).
Lee, E. et al. Tracking the influence of thermal expansion and oxygen vacancies on the thermal stability of Ni‐rich layered cathode materials. Adv. Sci. 7, 1902413 (2020).
Ge, M. et al. Kinetic limitations in single‐crystal high‐nickel cathodes. Angew. Chem. Int. Ed. 60, 17350–17355 (2021).
Wei, Z. et al. Probing the thermal degradation mechanism of polycrystalline and single-crystal Li(Ni0.8Co0.1Mn0.1)O2 cathodes from the perspective of oxygen vacancy diffusion. Energy Storage Mater. 56, 495–505 (2023).
Baddour-Hadjean, R. & Pereira-Ramos, J.-P. Raman microspectrometry applied to the study of electrode materials for lithium batteries. Chem. Rev. 110, 1278–1319 (2010).
Flores, E., Novák, P. & Berg, E. J. In situ and operando Raman spectroscopy of layered transition metal oxides for Li-ion battery cathodes. Front. Energy Res. 6, 82 (2018).
Ohzuku, T., Ueda, A., Nagayama, M., Iwakoshi, Y. & Komori, H. Comparative study of LiCoO2, LiNi12Co12O2 and LiNiO2 for 4 volt secondary lithium cells. Electrochim. Acta 38, 1159–1167 (1993).
Cui, Z., Guo, Z. & Manthiram, A. Assessing the intrinsic roles of key dopant elements in high-nickel layered oxide cathodes in lithium-based batteries. Adv. Energy Mater. 13, 2203853 (2023).
Tayal, A. et al. In situ insights into cathode calcination for predictive synthesis: kinetic crystallization of LiNiO2 from hydroxides. Adv. Mater. 36, 2312027 (2024).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy (EERE), Office of Vehicle Technologies of the US Department of Energy through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) award number DE-AC05-76RLO1830 (Z.C. and A.M.) and the Welch Foundation grant F-1254 (C.L. and A.M.). The synchrotron characterization work was supported by the US Department of Energy EERE, Vehicle Technologies Office, under Contract No. DE-AC02-06CH11357 (F.W.). We would like to thank S. Lee, S. Kmiec, A. Tayal and D. Wu for their assistance in the single-crystal cathode synthesis, Raman spectroscopy data collection, in situ-heating XRD data processing and ARC data collection, respectively. We thank R. Sim for insightful discussions.
Author information
Authors and Affiliations
Contributions
Z.C., C.L. and A.M. conceived the idea and designed the experiments. Z.C. and C.L. performed the synthesis of the materials, DSC and TGA measurements and characterized the physico-chemical properties of the other materials. F.W. performed the in situ heating XRD experiments. All authors wrote the paper. A.M. supervised the work.
Corresponding author
Ethics declarations
Competing interests
A.M. is a co-founder of TexPower EV Technologies, a company focusing on cobalt-free cathode materials for lithium-based batteries. The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Yaxiang Lu, Jinbao Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–50, Tables 1–4, Notes 1–9 and Refs. 1–60.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Z., Liu, C., Wang, F. et al. Navigating thermal stability intricacies of high-nickel cathodes for high-energy lithium batteries. Nat Energy 10, 490–501 (2025). https://doi.org/10.1038/s41560-025-01731-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01731-x
This article is cited by
-
Electronegativity and entropy design of layered oxides for sodium-ion batteries
Nature Communications (2026)
-
Metrics for evaluating safe electrolytes in energy-dense lithium batteries
Nature Energy (2025)
-
Designing safe and long-life lithium-ion batteries via a solvent-relay strategy
Nature Energy (2025)
-
Decoding thermal stability
Nature Energy (2025)
-
A transformer guided multi modal learning framework for predictive and causal assessment of thermal runaway in high energy batteries
Scientific Reports (2025)