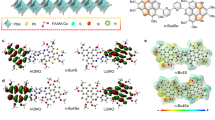
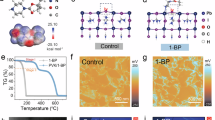
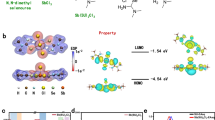
Abstract
Liquid-state 4-tert-butylpyridine is essential for achieving high performance in n–i–p perovskite solar cells. 4-tert- Butylpyridine effectively dissolves the lithium bis(trifluoromethanesulfonyl)imide dopant and stabilizes lithium ions. However, its high volatility and corrosive nature can degrade the perovskite layer and promote the formation of byproducts and pinholes in the hole transport layer under thermal stress, ultimately compromising device stability. Here we introduce a non-volatile, solid-state alternative—4-(N-carbazolyl)pyridine (4CP)—which stabilizes lithium ions and facilitates the formation of lithium bis(trifluoromethanesulfonyl)imide complexes. Perovskite solar cells incorporating 4CP achieve a power conversion efficiency of 26.2% (25.8% certified) and maintain 80% of their initial performance for over 3,000 h at maximum power point tracking. The unencapsulated devices retain 90% of their initial efficiency after 200 thermal shock cycles between −80 °C and 80 °C, and under continuous exposure to 65 °C and 85 °C. The adoption of 4CP could help improve the stability of n–i–p perovskite solar cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
McMeekin, D. P. et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 351, 151–155 (2016).
Saliba, M. et al. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 9, 1989–1997 (2016).
Jeon, N. J. et al. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 3, 682–689 (2018).
Yoo, J. J. et al. Efficient perovskite solar cells via improved carrier management. Nature 590, 587–593 (2021).
Turren-Cruz, S.-H., Hagfeldt, A. & Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 362, 449–453 (2018).
Jeong, J. et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021).
Park, J. et al. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 616, 724–730 (2023).
Kim, J. et al. Susceptible organic cations enable stable and efficient perovskite solar cells. Joule 9, 101879 (2025).
Chen, H. et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 384, 189–193 (2024).
Peng, W. et al. Reducing nonradiative recombination in perovskite solar cells with a porous insulator contact. Science 379, 683–690 (2023).
Li, G. et al. Highly efficient p–i–n perovskite solar cells that endure temperature variations. Science 379, 399–403 (2023).
Li, B. et al. Highly efficient and scalable p–i–n perovskite solar cells enabled by poly-metallocene interfaces. J. Am. Chem. Soc. 146, 13391–13398 (2024).
Li, Z. et al. Stabilized hole-selective layer for high-performance inverted p–i–n perovskite solar cells. Science 382, 284–289 (2023).
Yang, W. S. et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017).
Jung, E. H. et al. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 567, 511–515 (2019).
Min, H. et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 598, 444–450 (2021).
Kim, C. et al. Trimming defective perovskite layer surfaces for high-performance solar cells. Energy Environ. Sci. 17, 8582–8592 (2024).
Min, H. et al. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 366, 749–753 (2019).
Rombach, F. M., Haque, S. A. & Macdonald, T. J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 14, 5161–5190 (2021).
Ouedraogo, N. A. N. et al. Oxidation of spiro-OMeTAD in high-efficiency perovskite solar cells. ACS Appl. Mater. Interfaces 14, 34303–34327 (2022).
Hawash, Z., Ono, L. K. & Qi, Y. Moisture and oxygen enhance conductivity of LiTFSI-doped spiro-MeOTAD hole transport layer in perovskite solar cells. Adv. Mater. Interfaces 3, 1600117 (2016).
Juarez-Perez, E. J. et al. Role of the dopants on the morphological and transport properties of spiro-MeOTAD hole transport layer. Chem. Mater. 28, 5702–5709 (2016).
Wang, S. et al. Unveiling the role of tBP–LiTFSI complexes in perovskite solar cells. J. Am. Chem. Soc. 140, 16720–16730 (2018).
Jena, A. K., Ikegami, M. & Miyasaka, T. Severe morphological deformation of spiro-OMeTAD in (CH3NH3)PbI3 solar cells at high temperature. ACS Energy Lett. 2, 1760–1761 (2017).
Shin, Y. S. et al. De-doping engineering for efficient and heat-stable perovskite solar cells. Joule 9, 101779 (2025).
Al-Ashouri, A. et al. Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells. Energy Environ. Sci. 12, 3356–3369 (2019).
Jegorovė, A. et al. Starburst carbazole derivatives as efficient hole transporting materials for perovskite solar cells. Sol. RRL 6, 2100877 (2022).
Radhakrishna, K., Manjunath, S. B., Devadiga, D., Chetri, R. & Nagaraja, A. T. Review on carbazole-based hole transporting materials for perovskite solar cell. ACS Appl. Energy Mater. 6, 3635–3664 (2023).
Puerto Galvis, C. E., González Ruiz, D. A., Martínez-Ferrero, E. & Palomares, E. Challenges in the design and synthesis of self-assembling molecules as selective contacts in perovskite solar cells. Chem. Sci. 15, 1534–1556 (2024).
Wang, S. et al. Role of 4-tert-butylpyridine as a hole transport layer morphological controller in perovskite solar cells. Nano Lett. 16, 5594–5600 (2016).
Habisreutinger, S. N., Noel, N. K., Snaith, H. J. & Nicholas, R. Investigating the role of 4-tert-butylpyridine in perovskite solar cells. Adv. Energy Mater. 7, 1601079 (2017).
Kim, G. et al. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 370, 108–112 (2020).
Kim, M. et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 3, 2179–2192 (2019).
Duijnstee, E. A. et al. Understanding the degradation of methylenediammonium and its role in phase-stabilizing formamidinium lead triiodide. J. Am. Chem. Soc. 145, 10275–10284 (2023).
Tian, L. et al. Divalent cation replacement strategy stabilizes wide-bandgap perovskite for Cu(In,Ga)Se2 tandem solar cells. Nat. Photon. 19, 479–485 (2025).
Shibayama, N. et al. Control of molecular orientation of spiro-OMeTAD on substrates. ACS Appl. Mater. Interfaces 12, 50187–50191 (2020).
Coropceanu, V. et al. Charge transport in organic semiconductors. Chem. Rev. 107, 926–952 (2007).
Malinauskas, T. et al. Enhancing thermal stability and lifetime of solid-state dye-sensitized solar cells via molecular engineering of the hole-transporting material spiro-OMeTAD. ACS Appl. Mater. Interfaces 7, 11107–11116 (2015).
Sanehira, E. M. et al. Influence of electrode interfaces on the stability of perovskite solar cells: reduced degradation using MoOx/Al for hole collection. ACS Energy Lett. 1, 38–45 (2016).
Ye, L. et al. Superoxide radical derived metal-free spiro-OMeTAD for highly stable perovskite solar cells. Nat. Commun. 15, 7889 (2024).
Cho, Y. et al. Elucidating mechanisms behind ambient storage-induced efficiency improvements in perovskite solar cells. ACS Energy Lett. 6, 925–933 (2021).
Shen, Y., Deng, K., Chen, Q., Gao, G. & Li, L. Crowning lithium ions in hole-transport layer toward stable perovskite solar cells. Adv. Mater. 34, 2200978 (2022).
Laoire, C. O., Mukerjee, S., Abraham, K. M., Plichta, E. J. & Hendrickson, M. A. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium−air battery. J. Phys. Chem. C 114, 9178–9186 (2010).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat. Chem. 7, 50–56 (2015).
Lamberti, F. et al. Evidence of spiro-OMeTAD de-doping by tert-butylpyridine additive in hole-transporting layers for perovskite solar cells. Chem 5, 1806–1817 (2019).
Kim, S. et al. Enhancing thermal stability of perovskite solar cells through thermal transition and thin film crystallization engineering of polymeric hole transport layers. ACS Energy Lett. 9, 4501–4508 (2024).
Yang, J. & Kelly, T. L. Decomposition and cell failure mechanisms in lead halide perovskite solar cells. Inorg. Chem. 56, 92–101 (2017).
Divitini, G. et al. In situ observation of heat-induced degradation of perovskite solar cells. Nat. Energy 1, 15012 (2016).
Li, H. et al. Dynamics parameter correction for predicting the long-term stability of organic photovoltaics. Macromolecules 57, 6548–6558 (2024).
Hawash, Z., Ono, L. K., Raga, S. R., Lee, M. V. & Qi, Y. Air-exposure induced dopant redistribution and energy level shifts in spin-coated spiro-MeOTAD films. Chem. Mater. 27, 562–569 (2015).
Pan, H. et al. Advances in design engineering and merits of electron transporting layers in perovskite solar cells. Mater. Horiz. 7, 2276–2291 (2020).
Yao, Y. et al. Organic hole-transport layers for efficient, stable, and scalable inverted perovskite solar cells. Adv. Mater. 34, 2203794 (2022).
Dong, Y. et al. Dopant-induced interactions in spiro-OMeTAD: advancing hole transport for perovskite solar cells. Mater. Sci. Eng. R 162, 100875 (2025).
Euvrard, J. et al. p-Type molecular doping by charge transfer in halide perovskite. Mater. Adv. 2, 2956–2965 (2021).
You, S. et al. Bifunctional hole-shuttle molecule for improved interfacial energy level alignment and defect passivation in perovskite solar cells. Nat. Energy 8, 515–525 (2023).
Li, W. et al. Montmorillonite as bifunctional buffer layer material for hybrid perovskite solar cells with protection from corrosion and retarding recombination. J. Mater. Chem. A 2, 13587–13592 (2014).
Domanski, K. et al. Migration of cations induces reversible performance losses over day/night cycling in perovskite solar cells. Energy Environ. Sci. 10, 604–613 (2017).
Christians, J. A. et al. Tailored interfaces of unencapsulated perovskite solar cells for >1,000 hour operational stability. Nat. Energy 3, 68–74 (2018).
Khenkin, M. V. et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 5, 35–49 (2020).
Lin, Y.-H. et al. A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science 369, 96–102 (2020).
Lang, F. et al. Radiation hardness and self-healing of perovskite solar cells. Adv. Mater. 28, 8726–8731 (2016).
Tu, Y. et al. Perovskite solar cells for space applications: progress and challenges. Adv. Mater. 33, 2006545 (2021).
Miyazawa, Y. et al. Tolerance of perovskite solar cell to high-energy particle irradiations in space environment. iScience 2, 148–155 (2018).
Yang, J., Bao, Q., Shen, L. & Ding, L. Potential applications for perovskite solar cells in space. Nano Energy 76, 105019 (2020).
Dong, B. et al. Self-assembled bilayer for perovskite solar cells with improved tolerance against thermal stresses. Nat. Energy 10, 342–353 (2025).
Bulut, M. Thermal design, analysis, and testing of the first Turkish 3U communication CubeSat in low earth orbit. J. Therm. Anal. Calorim. 143, 4341–4353 (2021).
Lamb, D. A., Irvine, S. J. C., Baker, M. A., Underwood, C. I. & Mardhani, S. Thin film cadmium telluride solar cells on ultra-thin glass in low earth orbit—3 years of performance data on the AlSat-1N CubeSat mission. Prog. Photovolt. Res. Appl. 29, 1000–1007 (2021).
McAndrews, G. R. et al. Why perovskite thermal stress is unaffected by thin contact layers. Adv. Energy Mater. 14, 2400764 (2024).
Valiev, M. et al. NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 181, 1477–1489 (2010).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS All-Atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Jensen, K. P. & Jorgensen, W. L. Halide, ammonium, and alkali metal ion parameters for modeling aqueous solutions. J. Chem. Theory Comput. 2, 1499–1509 (2006).
Yeh, I.-C. & Berkowitz, M. L. Ewald summation for systems with slab geometry. J. Chem. Phys. 111, 3155–3162 (1999).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00338765, 2021R1A2C3004202), the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (RS-2025-02309702) and the Nano and Material Technology Development Program through the NRF funded by the Ministry of Science and ICT (RS-2025-25442266).
Author information
Authors and Affiliations
Contributions
C.Y., H.M. and S.Y. conceived the idea. K.K. fabricated the PSCs and performed characterization of the perovskite films. S.Y. synthesized the 4CP and performed the related characterizations. C.K. carried out the model PSC fabrication. Jeewon Park carried out PL characterizations. S.J. carried out TGA and DSC characterization. Y.K. and Jinsoo Park performed SEM and conductivity measurement. Z.S. carried out in situ PL measurement. M.K. conducted ATR-FTIR measurements. B.J.K. carried out PLQY measurements. J.O. and J.S.Y. performed the thermal shock test. C.Y. supervised the project. S.-J.S. performed the theoretical simulation. The paper was mainly written by S.-J.S., C.Y. and H.M., and all authors commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Thomas J. Macdonald, Jovana Milic and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19 and Tables 1 and 2.
Supplementary Data
Source Data for Supplementary Fig. 18.
Source data
Source Data Fig. 2
Statistical source J–V data.
Source Data Fig. 5
Statistical source J–V data and source J–V curve.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, K., Yang, S., Kim, C. et al. Non-volatile solid-state 4-(N-carbazolyl)pyridine additive for perovskite solar cells with improved thermal and operational stability. Nat Energy 10, 1427–1438 (2025). https://doi.org/10.1038/s41560-025-01864-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01864-z
This article is cited by
-
Additives for thermal stability
Nature Energy (2025)