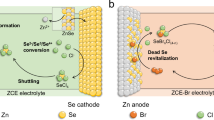
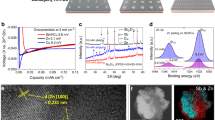
Abstract
Flow batteries are promising for renewable energy storage due to their safety and scalability. Zinc/bromine flow batteries (Zn/Br) are popular due to their high energy densities and inexpensive electrolytes. However, they have a poor service life and lead to environmental harm as a result of the generated corrosive and volatile Br2. Here we introduce a Br2 scavenger to the catholyte, reducing the Br2 concentration to an acceptable level (~7 mM). The scavenger, sodium sulfamate (SANa), reacts rapidly with Br2 to form a mild product, N-bromo sodium sulfamate (Br-SANa; Br+). Additionally, the two-electron transfer reaction of Br-SANa/Br− (Br+/Br−) increases the energy density. We have developed a Zn/Br flow battery, paired with a Zn anode, that outperforms traditional Zn/Br flow batteries in energy density (152 Wh l−1 versus 90 Wh l−1) and cycle life (>600 versus 30 cycles), using a sulfonated polyetheretherketone membrane. We assembled a 5-kW stack that operated stably for over 700 cycles (~1,400 h). Using this reaction, we have built a large-scale battery system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are included in the paper and its Supplementary Information. Source data are provided with this paper.
References
Tang, L. Y., Lu, W. J. & Li, X. F. Electrolytes for bromine-based flow batteries: challenges, strategies and prospects. Energy Stor. Mater. 70, 103532 (2024).
Mahmood, A., Zheng, Z. & Chen, Y. Zinc–bromine batteries: challenges, prospective solutions and future. Adv. Sci. 11, 2305561 (2024).
Popat, Y. et al. Carbon materials as positive electrodes in bromine-based flow batteries. ChemPlusChem 87, e202100441 (2022).
Futamata, M. & Takeuchi, T. Deterioration mechanism of the carbon-plastic electrode of the Zn-Br2 battery. Carbon 30, 1047–1053 (1992).
Saikia, I., Borah, A. J. & Phukan, P. Use of bromine and bromo-organic compounds in organic synthesis. Chem. Rev. 116, 6837–7042 (2016).
Kim, R. et al. Ultrathin Nafion-filled porous membrane for zinc/bromine redox flow batteries. Sci. Rep. 7, 10503 (2017).
Shakil, S. et al. Behavioral and neuronal effects of inhaled bromine gas: oxidative brain stem damage. Int. J. Mol. Sci. 22, 6316 (2021).
Carel, R. S., Belmaker, I., Potashnik, G., Levine, M. & Blau, R. Delayed health sequelae of accidental exposure to bromine gas. J. Toxicol. Environ. Health 36, 273–277 (1992).
Tang, L., Li, T., Lu, W. & Li, X. Reversible solid bromine complexation into Ti3C2Tx MXene carriers: a highly active electrode for bromine-based flow batteries with ultralow self-discharge. Energy Environ. Sci. 17, 3136–3145 (2024).
Zhu, F., Guo, W. & Fu, Y. Functional materials for aqueous redox flow batteries: merits and applications. Chem. Soc. Rev. 52, 8410–8446 (2023).
Wei, C., Song, J., Wang, Y., Tang, X. & Liu, X. Recent development of aqueous multivalent-ion batteries based on conversion chemistry. Adv. Funct. Mater. 33, 2304223 (2023).
Zhao, M. et al. A choline-based antifreezing complexing agent with selective compatibility for Zn–Br2 flow batteries. Small 20, 2307627 (2023).
Xu, C. et al. Practical high-energy aqueous zinc-bromine static batteries enabled by synergistic exclusion-complexation chemistry. Joule 8, 461–481 (2024).
Djerassi, C. Brominations with N-bromosuccinimide and related compounds; the Wohl-Ziegler reaction. Chem. Rev. 43, 271–317 (1948).
Choi, G. et al. Soft-hard zwitterionic additives for aqueous halide flow batteries. Nature 635, 89–95 (2024).
Eraković, M., Cinčić, D., Molčanov, K. & Stilinović, V. A crystallographic charge density study of the partial covalent nature of strong N⋅⋅⋅Br halogen bonds. Angew. Chem. Int. Ed. 58, 15702–15706 (2019).
Elroby, S. A. K., Noamaan, M. A. & Shibl, M. F. Quantum mechanical studies of the protonation and N-Br bond dissociation of the biologically important N-bromosuccinimide. J. Mol. Struct. THEOCHEM 915, 93–97 (2009).
Basha, S. J. & Chamundeeswari, S. P. V. Quantum computational, spectroscopic and molecular docking studies of 5,5-dimethylhydantoin and its bromine and chlorine derivatives. Chem. Data Collect. 29, 100461 (2020).
Panikar, S. S., Guirgis, G. A., Sheehan, T. G., Durig, D. T. & Durig, J. R. Infrared spectra, vibrational assignment, and ab initio calculations for N-bromo-hexafluoro-2-propanimine. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 90, 118–124 (2012).
Muthusubramanian, P. & Raj, A. S. Raman spectra of sodium sulphamate single crystal. Can. J. Chem. 61, 2048–2052 (1983).
Chen, X. et al. Raman spectroscopic investigation of tetraethylammonium polybromides. Inorg. Chem. 49, 8684–8689 (2010).
Yang, C. et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite. Nature 569, 245–250 (2019).
Aguirre, C. M., Kaspar, T. R., Radloff, C. & Halas, N. J. CTAB mediated reshaping of metallodielectric nanoparticles. Nano Lett. 3, 1707–1711 (2003).
Wang, H., Levin, C. S. & Halas, N. J. Nanosphere arrays with controlled sub-10-nm gaps as surface-enhanced Raman spectroscopy substrates. J. Am. Chem. Soc. 127, 14992–14993 (2005).
Sandford, C. et al. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 10, 6404–6422 (2019).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature 535, 406–410 (2016).
Tang, L. Y. et al. In situ vertically aligned MoS2 arrays electrodes for complexing agent-free bromine-based flow batteries with high power density and long lifespan. Adv. Energy Mater. 14, 2303282 (2024).
Biswas, S. et al. Minimal architecture zinc-bromine battery for low cost electrochemical energy storage. Energy Environ. Sci. 10, 114–120 (2017).
Li, X., Xie, C., Li, T., Zhang, Y. & Li, X. Low-cost titanium-bromine flow battery with ultrahigh cycle stability for grid-scale energy storage. Adv. Mater. 32, e2005036 (2020).
Eustace, D. J. Bromine complexation in zinc-bromine circulating batteries. J. Electrochem. Soc. 127, 528–532 (1980).
Bryans, D., McMillan, B. G., Spicer, M., Wark, A. & Berlouis, L. Complexing additives to reduce the immiscible phase formed in the hybrid ZnBr2 flow battery. J. Electrochem. Soc. 164, A3342–A3348 (2017).
Yuan, Z. et al. Low-cost hydrocarbon membrane enables commercial-scale flow batteries for long-duration energy storage. Joule 6, 884–905 (2022).
Waters, S. E., Robb, B. H. & Marshak, M. P. Effect of chelation on iron-chromium redox flow batteries. ACS Energy Lett. 5, 1758–1762 (2020).
Robertson, G. P. et al. Casting solvent interactions with sulfonated poly(ether ether ketone) during proton exchange membrane fabrication. J. Membr. Sci. 219, 113–121 (2003).
Frisch. M. J. et al. Gaussian 16 Rev. A.03 (Gaussian, 2016).
Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 25, 1463–1473 (2004).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Johnson, E. R. & Becke, A. D. A post-Hartree-Fock model of intermolecular interactions: inclusion of higher-order corrections. J. Chem. Phys. 124, 174104 (2006).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Xu, Y., Xie, C., Li, T. & Li, X. A high energy density bromine-based flow battery with two-electron transfer. ACS Energy Lett. 7, 1034–1039 (2022).
Wu, W., Luo, J., Wang, F., Yuan, B. & Liu, T. L. A self-trapping, bipolar viologen bromide electrolyte for redox flow batteries. ACS Energy Lett. 6, 2891–2897 (2021).
Liu, W. et al. A highly stable neutral viologen/bromine aqueous flow battery with high energy and power density. Chem. Commun. 55, 4801–4804 (2019).
Lin, G. et al. Advanced hydrogen-bromine flow batteries with improved efficiency, durability and cost. J. Electrochem. Soc. 163, A5049–A5056 (2015).
Huskinson, B. et al. A metal-free organic-inorganic aqueous flow battery. Nature 505, 195–198 (2014).
Abunaeva, L. et al. Successful charge-discharge experiments of anthraquinone-bromate flow battery: first report. Energies 15, 7967 (2022).
Zeng, Y., Yang, Z., Lu, F. & Xie, Y. A novel tin-bromine redox flow battery for large-scale energy storage. Appl. Energy 255, 113756 (2019).
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (grant no. 22525081 to X.L., 22209179 to C.X. and 22478379 to C.X.), the International Partnership Program of the Chinese Academy of Sciences (121421KYSB20210028 to X.L.), the Science and Technology Major Project of Liaoning Province (grant no. 2024JH1/11700011 to C.X.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA0400201 to X.L.), Liaoning Binhai Laboratory (no. LBLD 202401) and the Liaoning Provincial Natural Science Foundation (2023-MS-010 to C.X.). We thank A. L. Chun of Science Storylab for critically reading and editing the manuscript. We thank Y. Song and C. Yuan at the Dalian Institute of Chemical Physics for their support in system assembly. We thank Z. Zhao at the Dalian Institute of Chemical Physics for his support with SERS testing. We thank C. Mu at the Dalian Institute of Chemical Physics for his support with theoretical calculations.
Author information
Authors and Affiliations
Contributions
Y.X., C.X. and X.L. conceived the projects and designed the experiments. Y.X. and C.X. conducted the electrochemical tests and material characterizations. T.L. conducted the theoretical calculations. Y.X., C.X., Z.P. and X.L. co-wrote and revised the paper. C.X. and X.L. supervised the work and discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Yuan Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–42 and Tables 1–13.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Li, T., Peng, Z. et al. Grid-scale corrosion-free Zn/Br flow batteries enabled by a multi-electron transfer reaction. Nat Energy 10, 1470–1481 (2025). https://doi.org/10.1038/s41560-025-01907-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01907-5
This article is cited by
-
Sodium sulfamate breakthrough powers large-scale zinc/bromine flow batteries
Nature Reviews Electrical Engineering (2026)