Abstract
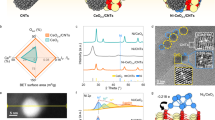
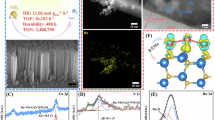
The Haber–Bosch process for ammonia synthesis contributes up to ~3% of global greenhouse gas emissions. Plasmonic catalysts strongly concentrate light and can alter the reaction intermediates via out-of-equilibrium processes, providing the potential for an alternative, less-energy-intensive pathway to synthesize ammonia. Here we show that gold-ruthenium (AuRu) bimetallic nanoparticles can synthesize ammonia at room temperature and pressure using visible light. We create AuRu alloys with varying compositions and achieve ammonia production rates of ~60 μmol per gram of catalyst bed per hour. In situ infrared spectroscopy reveals that light accelerates the hydrogenation of nitrogen intermediates compared to conventional thermal catalysis. Through computational modelling, we demonstrate that photo-excited electrons enable associative hydrogenation pathways for nitrogen activation rather than direct nitrogen–nitrogen bond breaking. This light-assisted mechanism requires both hydrogen and light working together to overcome the nitrogen activation barrier, mimicking how biological enzymes produce ammonia naturally and providing fundamental insights for developing sustainable, energy-efficient chemical synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are available via Zenodo at https://doi.org/10.5281/zenodo.14291695 (ref. 54). Source data are provided with this paper.
Code availability
Matlab codes, Lumerical FDTD files and the scripts for hot-carrier generation are available via Zenodo at https://doi.org/10.5281/zenodo.14291695 (ref. 54).
References
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636 (2008).
Miao, B., Zhang, L., Wu, S. & Chan, S. H. The economics of power generation and energy storage via solid oxide cell and ammonia. Int. J. Hydrogen Energy 47, 26827–26841 (2022).
Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018).
Smith, C., Hill, A. K. & Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 13, 331–344 (2020).
Yuan, L., Bourgeois, B. B., Carlin, C. C., da Jornada, F. H. & Dionne, J. A. Sustainable chemistry with plasmonic photocatalysts. Nanophotonics 12, 2745–2762 (2023).
Neugebauer, J. & Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B 46, 16067–16080 (1992).
Hu, C. et al. Surface plasmon enabling nitrogen fixation in pure water through a dissociative mechanism under mild conditions. J. Am. Chem. Soc. 141, 7807–7814 (2019).
Mao, C., Yu, L., Li, J., Zhao, J. & Zhang, L. Energy-confined solar thermal ammonia synthesis with K/Ru/TiO2-xHx. Appl. Catal. B 224, 612–620 (2018).
Mao, C. et al. Beyond the thermal equilibrium limit of ammonia synthesis with dual temperature zone catalyst powered by solar light. Chem 5, 2702–2717 (2019).
Li, X., Zhang, X., Everitt, H. O. & Liu, J. Light-induced thermal gradients in ruthenium catalysts significantly enhance ammonia production. Nano Lett. 19, 1706–1711 (2019).
Yang, J. et al. High-efficiency “working-in-tandem” nitrogen photofixation achieved by assembling plasmonic gold nanocrystals on ultrathin titania nanosheets. J. Am. Chem. Soc. 140, 8497–8508 (2018).
Aslam, U., Rao, V. G., Chavez, S. & Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 1, 656–665 (2018).
Zhou, L. A. et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 5, 61–70 (2020).
Zhang, Z.-Y. et al. Photo-enhanced dry reforming of methane over Pt-Au/P25 composite catalyst by coupling plasmonic effect. J. Catal. 413, 829–842 (2022).
Wang, J., Heo, J., Chen, C., Wilson, A. J. & Jain, P. K. Ammonia oxidation enhanced by photopotential generated by plasmonic excitation of a bimetallic electrocatalyst. Angew. Chem. Int. Ed. 59, 18430–18434 (2020).
Jiang, W. et al. Active site engineering on plasmonic nanostructures for efficient photocatalysis. ACS Nano 17, 4193–4229 (2023).
Wang, Y. et al. In situ investigation of ultrafast dynamics of hot electron-driven photocatalysis in plasmon-resonant grating structures. J. Am. Chem. Soc. 144, 3517–3526 (2022).
Hou, T. et al. Porous CuFe for plasmon-assisted N2 photofixation. ACS Energy Lett. 5, 2444–2451 (2020).
Puértolas, B., Comesaña-Hermo, M., Besteiro, L. V., Vázquez-González, M. & Correa-Duarte, M. A. Challenges and opportunities for renewable ammonia production via plasmon-assisted photocatalysis. Adv. Energy Mater. 12, 2103909 (2022).
Zhang, Q. et al. Selective control of fcc and hcp crystal structures in Au–Ru solid-solution alloy nanoparticles. Nat. Comm. 9, 510 (2018).
Zhang, Q. et al. Solid-solution alloy nanoparticles of a combination of immiscible Au and Ru with a large gap of reduction potential and their enhanced oxygen evolution reaction performance. Chem. Sci. 10, 5133–5137 (2019).
García-García, F. R., Guerrero-Ruiz, A. & Rodríguez-Ramos, I. Role of B5-type sites in Ru catalysts used for the NH3 decomposition reaction. Top. Catal. 52, 758–764 (2009).
Peng, S., Meng, A. C., Braun, M. R., Marshall, A. F. & McIntyre, P. C. Plasmons and inter-band transitions of hexagonal close packed gold nanoparticles. Appl. Phys. Lett. 115, 051107 (2019).
Ageev, V. N. Desorption induced by electronic-transitions. Prog. Surf. Sci. 47, 55–204 (1994).
Linic, S., Aslam, U., Boerigter, C. & Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 14, 567–576 (2015).
Bourgeois, B. et al. Linking atomic and reactor scale plasmon photocatalysis in acetylene hydrogenation with optically coupled ETEM. Microsc. Microanal. 29, 1298–1299 (2023).
Dionne, J. A. et al. Multicomponent alloyed plasmonic photocatalysis. US Patent Application US 2023/0364597 A1, United States, 2023.
Yuan, L. et al. Morphology-dependent reactivity of a plasmonic photocatalyst. ACS Nano 14, 12054–12063 (2020).
Yuan, Y. et al. Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination. Science 378, 889–893 (2022).
Besteiro, L. V., Kong, X. T., Wang, Z. M., Hartland, G. & Govorov, A. O. Understanding hot-electron generation and plasmon relaxation in metal nanocrystals: quantum and classical mechanisms. ACS Photonics 4, 2759–2781 (2017).
Brown, A. M., Sundararaman, R., Narang, P., Goddard, W. A. 3rd & Atwater, H. A. Nonradiative plasmon decay and hot carrier dynamics: effects of phonons, surfaces, and geometry. ACS Nano 10, 957–966 (2016).
Solovyev, I. V. & Imada, M. Screening of Coulomb interactions in transition metals. Phys. Rev. B 71, 045103 (2005).
Sayan, Ş, Kantcheva, M., Suzer, S. & Uner, D. O. FTIR characterization of Ru/SiO2 catalyst for ammonia synthesis. J. Mol. Struct. 480-481, 241–245 (1999).
Mehta, P. et al. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 1, 269–275 (2018).
Vojvodic, A. et al. Exploring the limits: a low-pressure, low-temperature Haber–Bosch process. Chem. Phys. Lett. 598, 108–112 (2014).
Fang, H. et al. Challenges and opportunities of Ru-based catalysts toward the synthesis and utilization of ammonia. ACS Catal. 12, 3938–3954 (2022).
Ohki, Y. et al. Nitrogen reduction by the Fe sites of synthetic [Mo3S4Fe] cubes. Nature 607, 86–90 (2022).
Vegge, T. & Cheng, P. Ammonia synthesis by ternary ruthenium complex hydrides. Nat. Catal. 4, 989–990 (2021).
Zheng, J. et al. Efficient non-dissociative activation of dinitrogen to ammonia over lithium-promoted ruthenium nanoparticles at low pressure. Angew. Chem. Int. Ed. 58, 17335–17341 (2019).
Shetty, S., Jansen, A. P. J. & van Santen, R. A. Active sites for N2 dissociation on ruthenium. J. Phys. Chem. C. 112, 17768–17771 (2008).
Threatt, S. D. & Rees, D. C. Biological nitrogen fixation in theory, practice, and reality: a perspective on the molybdenum nitrogenase system. FEBS Lett. 597, 45–58 (2023).
Vader, D. T., Viskanta, R. & Incropera, F. P. Design and testing of a high-temperature emissometer for porous and particulate dielectrics. Rev. Sci. Instrum. 57, 87–93 (1986).
Lou, M. et al. Direct H2S decomposition by plasmonic photocatalysis: efficient remediation plus sustainable hydrogen production. ACS Energy Lett. 7, 3666–3674 (2022).
Robatjazi, H. et al. Plasmon-driven carbon–fluorine (C(sp3)–F) bond activation with mechanistic insights into hot-carrier-mediated pathways. Nat. Catal. 3, 564–573 (2020).
Zhou, L. et al. Hot carrier multiplication in plasmonic photocatalysis. Proc. Natl Acad. Sci. USA 118, e2022109118 (2021).
Zhao, Y. et al. Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates?. Adv. Sci. 6, 1802109 (2019).
Zhang, C. et al. Atomic molybdenum for synthesis of ammonia with 50% Faradic efficiency. Small 18, e2106327 (2022).
Qiu, W. et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Comm. 9, 3485 (2018).
Daneshfar, N. Effect of interparticle plasmon coupling and temperature on the optical properties of bimetallic composite nanoparticles with a core-shell structure. J. Appl. Phys. 117, 123105 (2015).
Malasi, A. et al. Enhanced and tunable optical quantum efficiencies from plasmon bandwidth engineering in bimetallic CoAg nanoparticles. APL Photon. 1, 076101 (2016).
Cortés-López, S. et al. Berreman effect in bimetallic nanolayered metamaterials. Opt. Mater. 99, 109578 (2020).
Johnson, P. B. & Christy, R. W. Optical constants of the noble metals. Phys. Rev. B 6, 4370–4379 (1972).
Wojcik, H. et al. Physical characterization of PECVD and PEALD Ru (-C) films and comparison with PVD ruthenium film properties. J. Electrochem. Soc. 159, H166 (2011).
Yuan, L. et al. Atmospheric pressure ammonia synthesis on AuRu catalysts enabled by plasmon-controlled hydrogenation and nitrogen-species desorption. Zenodo https://doi.org/10.5281/zenodo.14291695 (2024).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Becke, A. D. & Johnson, E. R. A density-functional model of the dispersion interaction. J. Chem. Phys. 123, 154101 (2005).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Makov, G. & Payne, M. C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B 51, 4014–4022 (1995).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Govind, N., Wang, Y. A., da Silva, A. J. R. & Carter, E. A. Accurate ab initio energetics of extended systems via explicit correlation embedded in a density functional environment. Chem. Phys. Lett. 295, 129–134 (1998).
Huang, C., Pavone, M. & Carter, E. A. Quantum mechanical embedding theory based on a unique embedding potential. J. Chem. Phys. 134, 154110 (2011).
Libisch, F., Huang, C. & Carter, E. A. Embedded correlated wavefunction schemes: theory and applications. Acc. Chem. Res. 47, 2768–2775 (2014).
Yu, K., Krauter, C. M., Dieterich, J. M. & Carter, E. A. Density and Potential Functional Embedding: Theory and Practice Fragmentation: Toward Accurate Calculations on Complex Molecular Systems (John Wiley & Sons, 2017).
Widmark, P. O., Malmqvist, P. Å & Roos, B. O. Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions. I. First row atoms. Theor. Chim. Acta 77, 291–306 (1990).
Roos, B. O., Lindh, R., Malmqvist, P. Å, Veryazov, V. & Widmark, P. O. Main group atoms and dimers studied with a new relativistic ANO basis set. J. Phys. Chem. A 108, 2851–2858 (2004).
Roos, B. O., Lindh, R., Malmqvist, P. A., Veryazov, V. & Widmark, P. O. New relativistic ANO basis sets for transition metal atoms. J. Phys. Chem. A 109, 6575–6579 (2005).
Andersson, K., Malmqvist, P. Å & Roos, B. O. Second-order perturbation theory with a complete active space self-consistent field reference function. J. Chem. Phys. 96, 1218–1226 (1992).
Andersson, K., Malmqvist, P. A., Roos, B. O., Sadlej, A. J. & Wolinski, K. Second-order perturbation theory with a CASSCF reference function. J. Phys. Chem. 94, 5483–5488 (2002).
Ghigo, G., Roos, B. O. & Malmqvist, P. -Å A modified definition of the zeroth-order Hamiltonian in multiconfigurational perturbation theory (CASPT2). Chem. Phys. Lett. 396, 142–149 (2004).
Roos, B. O., Taylor, P. R. & Sigbahn, P. E. M. A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem. Phys. 48, 157–173 (1980).
Malmqvist, P. Å, Rendell, A. & Roos, B. O. The restricted active space self-consistent-field method, implemented with a split graph unitary group approach. J. Phys. Chem. 94, 5477–5482 (1990).
Werner, H. J. & Meyer, W. A quadratically convergent MCSCF method for the simultaneous optimization of several states. J. Chem. Phys. 74, 5794–5801 (1981).
Forsberg, N. & Malmqvist, P. Å. Multiconfiguration perturbation theory with imaginary level shift. Chem. Phys. Lett. 274, 196–204 (1997).
Aquilante, F. et al. Modern quantum chemistry with Molcas. J. Chem. Phys. 152, 214117 (2020).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
L.Y., B.B.B., Y.Z., A.X.D., A.S.M.-G. and J.A.D. acknowledge the support from the Keck Foundation under grant number 994816, the support from the ATW–Alan T Waterman Award from the National Science Foundation (NSF) under grant number 1933624 and the support from the NSF Center for Adopting Flaws as Features (NSF CHE-2124983). L.Y., B.B.B., Y.Z., A.X.D., A.S.M.-G. Yi Cui (0000-0001-8219-1856), K.X., Y.W., Yi Cui (0000-0002-6103-6352), A.M. and J.A.D. acknowledge the Office of Basic Energy Sciences, US Department of Energy, Division of Materials Science and Engineering, DE-AC02-76SF00515. L.Y. and J.A.D. acknowledge the support from the National Research Foundation of Korea (NRF) grants funded by the Korean government (Ministry of Science and Information and Communication Technology (ICT)) (number RS-2024-00421181). K.X., Y.W. and A.M. acknowledge the support from the Office of Naval Research MURI Award N00014-21-1-2377. J.L.B. acknowledges the financial support provided by the American Chemical Society Petroleum Research Fund (PRF number 65744-DNI6). In addition, J.L.B. thanks the Boston College Linux Cluster Center for cluster computing resources. B.B.B. was supported by the National Science Foundation Graduate Research Fellowship under grant number DGE-1656518. L.Y. and J.A.D. acknowledge the use and support of the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation under award ECCS-2026822. This work utilized beamline 4-1 at the Stanford Synchrotron Radiation Lightsource (SSRL) and beamline 7-BM (QAS) at the National Synchrotron Light Source II (NSLS-II), both of which are US Department of Energy Office of Science User Facilities. L.Y. acknowledges the helpful support and discussion regarding X-ray absorption (XAS) measurements from D. Yang and L. Ma from Brookhaven National Laboratory. L.Y. acknowledges the helpful discussion regarding synthesis and collection of AuRu bimetallic alloy with Q. Zhang from Kyoto University, Japan.
Author information
Authors and Affiliations
Contributions
L.Y. and J.A.D. conceptualized the research, including the design of electromagnetic simulations and experiments. L.Y., B.B.B., A.X.D. and Z.C. finalized the synthetic protocol and experimental details under the supervision of M.R.J. and J.A.D. L.Y., B.B.B., A.X.D., A.S.M.-G., Yi Cui (0000-0001-8219-1856), K.X. and Y.W. conducted all TEM imaging and analysis, supervised by Yi Cui (0000-0002-6103-6352), A.M. and J.A.D. L.Y. performed the electromagnetic simulations and calculations. E.B. and J.L.B. carried out the first-principle quantum mechanical (QM) calculations, ECW excited-state calculations and provided insights into reaction pathways. L.Y. and Y.Z. conducted the in situ DRIFTS measurements and analysis, whereas L.Y. and Z.X. performed the synchrotron X-ray absorption measurements. All authors contributed to discussions, provided insights and participated in manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
L.Y., B.B.B., A.X.D. and J.A.D. declare that a US patent application for the multicomponent alloyed plasmonic photocatalytic properties is pending (US 18/196, 359). The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Alberto Naldoni and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Figs. 1–36 and Notes 1 and 2.
Supplementary Data 1
Supplementary data for Supplementary Figs. 11, 31a,c and 35.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, L., Bourgeois, B.B., Begin, E. et al. Atmospheric-pressure ammonia synthesis on AuRu catalysts enabled by plasmon-controlled hydrogenation and nitrogen-species desorption. Nat Energy 11, 98–108 (2026). https://doi.org/10.1038/s41560-025-01911-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01911-9