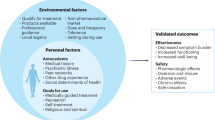
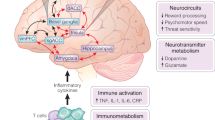
Abstract
There is a push to shrink the anticipated 17-year research-to-practice gap for psychedelic-assisted therapy (PAT), offering precarious hope to those with disabling mental health conditions. However, numerous questions regarding how PAT works, how well it works, for whom and in what context remain. Substantial changes to current systems of care, including regulatory approvals, clinical training and access will all be required to accommodate PAT, a multimodal therapy that combines pharmacological and psychotherapy components that are not routinely available outside clinical research settings. Implementation science can help to reduce the gap in a way that maintains scientific rigour by simultaneously examining the safety, effectiveness and implementation of PAT. Specifically, precision implementation science methods (for example, sequential multiple assignment randomized trial (SMART) designs), hybrid study designs, valid measurement of fidelity and use of theory-based models and frameworks for treatment development will accelerate the process of implementation while balancing safety and quality. The time to proceed, with accelerated caution, is now.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Feduccia, A. A. et al. Breakthrough for trauma treatment: safety and efficacy of MDMA-assisted psychotherapy compared to paroxetine and sertraline. Front. Psychiatry 10, 650 (2019).
Heal, D. J., Smith, S. L., Belouin, S. J. & Henningfield, J. E. Psychedelics: threshold of a therapeutic revolution. Neuropharmacology 236, 109610 (2023).
MindMed, MindMed receives FDA breakthrough therapy designation and announces positive 12-week durability data from phase 2B study of MM120 for generalized anxiety disorder. ir.mindmed.co https://ir.mindmed.co/news-events/press-releases/detail/137/mindmed-receives-fda-breakthrough-therapy-designation-and-announces-positive-12-week-durability-data-from-phase-2b-study-of-mm120-for-generalized-anxiety-disorder (MindMed, 2024).
APA. COVID-19 practitioner impact survey 2022. apa.org https://www.apa.org/pubs/reports/practitioner/2022-covid-psychologist-workload (American Psychological Association, 2022).
Viña, S. M. & Stephens, A. L. Psychedelics and workplace harm. Front. Psychiatry 14, 1186541 (2023).
Forstmann, M. & Sagioglou, C. Lifetime experience with (classic) psychedelics predicts pro-environmental behavior through an increase in nature relatedness. J. Psychopharmacol. 31, 975–988 (2017).
Forstmann, M. et al. Among psychedelic-experienced users, only past use of psilocybin reliably predicts nature relatedness. J. Psychopharmacol. 37, 93–106 (2023).
Reardon, S. FDA rejects ecstasy as a therapy: what’s next for psychedelics? Nature NEWS https://www.nature.com/articles/d41586-024-02597-x (13 August 2024).
Mitchell, J. M. et al. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat. Med. 29, 2473–2480 (2023).
Goodwin, G. M., Malievskaia, E., Fonzo, G. A. & Nemeroff, C. B. Must psilocybin always ‘assist psychotherapy’? Am. J. Psychiatry. 181, 20–25 (2024).
Psychedelic Drugs: Considerations for Clinical Investigations (United States Food and Drug Administration, 2023).
McGuire, A. L. et al. Developing an ethics and policy framework for psychedelic clinical care: a consensus statement. JAMA Netw. Open 7, e2414650 (2024).
Andrews, C. M., Hall, W., Humphreys, K. & Marsden, J. Crafting effective regulatory policies for psychedelics: what can be learned from the case of cannabis?. Addiction 120, 201–206 (2025).
Volkow, N. D., Gordon, J. A. & Wargo, E. M. Psychedelics as therapeutics—potential and challenges. JAMA Psychiatry. 80, 979–980 (2023).
Bara, A., Ferland, J. N., Rompala, G., Szutorisz, H. & Hurd, Y. L. Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 22, 423–438 (2021).
Testai, F. D. et al. Use of marijuana: effect on brain health: a scientific statement from the American Heart Association. Stroke 53, e176–e187 (2022).
Pacher, P., Steffens, S., Haskó, G., Schindler, T. H. & Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad and the ugly. Nat. Rev. Cardiol. 15, 151–166 (2018).
Fernandez, A. C. et al. Prevalence and frequency of cannabis use among adults ages 50-80 in the United States. Cannabis Cannabinoid Res 9, 59–64 (2024).
Siegel, J. S., Daily, J. E., Perry, D. A. & Nicol, G. E. Psychedelic drug legislative reform and legalization in the US. JAMA Psychiatry. 80, 77–83 (2023).
National Council for Mental Wellbeing. New study: behavioral health workforce shortage will negatively impact society. thenationalcouncil.org https://www.thenationalcouncil.org/news/help-wanted/ (25 April 2023).
The economics of administration action on student debt. whitehouse.gov https://www.whitehouse.gov/cea/written-materials/2024/04/08/the-economics-of-administration-action-on-student-debt/ (The White House, 2024).
Pleet, M. M., White, J., Zamaria, J. A. & Yehuda, R. Reducing the harms of nonclinical psychedelics use through a peer-support telephone helpline. Psychedelic Med. (New Rochelle) 1, 69–73 (2023).
Hearn, B. G., Brubaker, M. D. & Richardson, G. Counselors’ attitudes toward psychedelics and their use in therapy. J. Couns. Dev. 100, 364–373 (2022).
Kucsera, A., Suppes, T. & Haug, N. A. Psychologists’ and psychotherapists’ knowledge, attitudes and clinical practices regarding the therapeutic use of psychedelics. Clin. Psychol. Psychotherapy 30, 1369–1379 (2023).
Tai, S. J. et al. Development and evaluation of a therapist training program for psilocybin therapy for treatment-resistant depression in clinical research. Front. Psychiatry 12, 586682 (2021).
Brennan, W. & Belser, A. B. Models of psychedelic-assisted psychotherapy: a contemporary assessment and an introduction to EMBARK, a transdiagnostic, trans-drug model. Front. Psychol. 13, 866018 (2022).
Columbia School of Social Work. Psychedelic therapy training program online. columbia.edu https://pttp.socialwork.columbia.edu/ (Columbia Univ., accessed 24 June 2024).
Center for Mental Health Services Research, Washington University. Psychedelic-assisted therapies learning community (PAT LC) online. wustl.edu https://cmhsr.wustl.edu/research-projects/psychedelic-assisted-therapies-learning-community-pat-lc/?_ga=2.253513345.980339201.1719325568-530958589.1639675267 (Brown School at Washington Univ. in St Louis, accessed 24 June 2024).
Department of Health and Human Services. Dissemination and implementation research in health (R01) NIH funding opportunity: PAR-19-274. nih.gov https://grants.nih.gov/grants/guide/pa-files/PAR-19-274.html (NIH, 2018).
Aarons, G. A., Ehrhart, M. G. & Farahnak, L. R. The Implementation Leadership Scale (ILS): development of a brief measure of unit level implementation leadership. Implement. Sci. 9, 45 (2014).
Cabassa, L. J., Druss, B., Wang, Y. & Lewis-Fernández, R. Collaborative planning approach to inform the implementation of a healthcare manager intervention for Hispanics with serious mental illness: a study protocol. Implement. Sci. 6, 80 (2011).
Cabassa, L. J. et al. Using the collaborative intervention planning framework to adapt a health-care manager intervention to a new population and provider group to improve the health of people with serious mental illness. Implement. Sci. 9, 178 (2014).
Kirchner, J. E., Smith, J. L., Powell, B. J., Waltz, T. J. & Proctor, E. K. Getting a clinical innovation into practice: an introduction to implementation strategies. Psychiatry Res. 283, 112467 (2020).
Frank, H. E., Kemp, J., Benito, K. G. & Freeman, J. B. Precision implementation: an approach to mechanism testing in implementation research. Adm. Policy Ment. Health 49, 1084–1094 (2022).
Dopp, A. R. et al. Translating economic evaluations into financing strategies for implementing evidence-based practices. Implement. Sci. 16, 66 (2021).
Guise J. M. et al. Systematic Reviews of Complex Multicomponent Health Care Interventions (US Agency for Healthcare Research and Quality, 2014).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2019).
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 323, 844–853 (2020).
Siegel, J. S. et al. Psilocybin desynchronizes the human brain. Nature 632, 131–138 (2024).
Nicol, G. E. et al. A smartphone-based technique to detect dynamic user preferences for tailoring behavioral interventions: observational utility study of ecological daily needs assessment. JMIR Mhealth Uhealth 8, e18609 (2020).
Proctor, E. K. & Geng, E. A new lane for science. Science 374, 659 (2021).
Teague, G. B., Mueser, K. T. & Rapp, C. A. Advances in fidelity measurement for mental health services research: four measures. Psychiatr. Serv. 63, 765–771 (2012).
Beidas, R. S. et al. A randomized trial to identify accurate and cost-effective fidelity measurement methods for cognitive-behavioral therapy: project FACTS study protocol. BMC Psychiatry. 16, 323 (2016).
Lei, H., Nahum-Shani, I., Lynch, K., Oslin, D. & Murphy, S. A. A ‘SMART’ design for building individualized treatment sequences. Annu. Rev. Clin. Psychol. 8, 21–48 (2012).
Huynh, L., Toyserkani, G. A. & Morrato, E. H. Pragmatic applications of implementation science frameworks to regulatory science: an assessment of FDA Risk Evaluation and Mitigation Strategies (REMS) (2014-2018). BMC Health Serv. Res. 21, 779 (2021).
Tajchman, S. et al. Implementation and use of risk evaluation and mitigation strategies programs in practice: a scoping review of the literature. Appl. Clin. Inform. 13, 1151–1160 (2022).
Smith, M. Y., Gaglio, B. & Anatchkova, M. The use of implementation science theories, models, and frameworks in implementation research for medicinal products: a scoping review. Health Res. Policy Syst. 22, 17 (2024).
Luke, D. A. et al. The Translational Science Benefits Model: a new framework for assessing the health and societal benefits of clinical and translational sciences. Clin. Transl. Sci. 11, 77–84 (2018).
Ramsey, A. T. et al. Designing for Accelerated Translation (DART) of emerging innovations in health. J. Clin. Transl. Sci. 3, 53–58 (2019).
Proctor, E. et al. FAST: a framework to assess speed of translation of health innovations to practice and policy. Glob. Implement. Res. Appl. 2, 107–119 (2022).
Sarewitz, D. ‘Unknown knowns’. Issues Sci. Technol. 37, 18–19 (2020).
Gerritsen, S. et al. Community group model building as a method for engaging participants and mobilising action in public health. Int. J. Environ. Res. Public Health 17, 3457 (2020).
Johnston, C. B., Mangini, M., Grob, C. & Anderson, B. The safety and efficacy of psychedelic-assisted therapies for older adults: knowns and unknowns. Am. J. Geriatr. Psychiatry 31, 44–53 (2023).
Kettner, H., Roseman, L., Gazzaley, A., Carhart-Harris, R. & Pasquini, L. Improvements in well-being following naturalistic psychedelic use and underlying mechanisms of change in older adults: a prospective cohort study. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3977169/v1 (2024).
Acknowledgements
This work was made possible by National Institutes of Health (NIH) grants T32MH019960 and R25 MH112473, as well as the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. This work was also supported by research grants from the Center for Brain Research in Mood Disorders and the Taylor Family Foundation for Innovative Psychiatric Research, the Mallinckrodt Institute of Radiology, the McDonnell Center for Systems Neuroscience and the Institute for Clinical and Translational Sciences at Washington University School of Medicine in St Louis, and by the Barnes Jewish Hospital Foundation and Usona Institute.
Author information
Authors and Affiliations
Contributions
G.E.N. developed the overall concept for the manuscript, conducted an extensive review of the existing literature, created the content for figures, tables and text boxes, drafted the initial manuscript, and substantively revised it based on reviewer and editorial feedback. D.R.A. contributed extensively to the manuscript by providing explanatory details and historical background, and provided substantial revisions to improve its readability for a diverse readership. E.J.L. contributed to the overall conceptual design of the manuscript and provided substantial edits regarding pharmacological and regulatory content. L.J.C. worked closely with G.E.N. to devise the overall concept, providing guidance and content relevant to implementation science methods. All authors have reviewed, revised and approved the submitted version. All authors agree to be personally accountable for their contributions to this work and will ensure that questions related to accuracy or integrity are appropriately investigated, resolved and documented in the literature.
Corresponding author
Ethics declarations
Competing interests
G.E.N. has received salary support from institutional grants supported by the National Center for Translational Sciences (NCATS) and the National Institute of Digestive and Diabetes and Kidney Diseases (NIDDK), research support from the National Institute of Mental Health (NIMH), the Health Resources and Services Administration (HRSA), the Patient Centered Outcomes Research Institute (PCORI), the Barnes Jewish Hospital Foundation and the Institute for Clinical Translational Sciences, the Taylor Family Institute for Innovative Psychiatric Research, the Mallinckrodt Institute of Radiology, the McDonnell Center for Systems Neuroscience and the Center for Holistic Interdisciplinary Research in Psychedelics (CHIRP), funded by a Transcend Initiative Grant through the Here & Next Research Grants programme at Washington University. She has served as a co-investigator or principal investigator for studies funded by COMPASS Pathways, LB Pharmaceuticals, Inc., Usona Institute and Alkermes, Inc., and has received personal fees as a consultant from Carelon, Novartis and Alkermes, Inc. D.R.A. has received grant support from the NIMH (T32MH019960). E.J.L. has received grant support from Fast grants and Jansen, has consulting relationships with Pritikin ICR, Boehringer Ingelheim, Merck, IngenioRX and Prodeo and a patent pending for the use of fluvoxamine for COVID-19. L.J.C. has received research support from NIMH (T32MH019960), the Washington University in St Louis Institute for Public Health and the Center for Dissemination and Implementation (D&I) and the Center for Mental Health Services Research in the Brown School at Washington University.
Peer review
Peer review information
Nature Human Behaviour thanks Dominic Sisti, Matthew Wall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nicol, G.E., Adams, D.R., Lenze, E.J. et al. Shrinking the know–do gap in psychedelic-assisted therapy. Nat Hum Behav 9, 665–672 (2025). https://doi.org/10.1038/s41562-025-02103-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41562-025-02103-x