Abstract
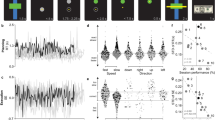
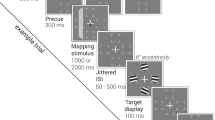
Adaptation is the process by which we adjust internal models of the body, world and mind in response to sensory feedback. Although adaptation is studied extensively in the context of motor control, there is limited evidence that cognitive functions such as working memory are subject to the same error-driven adaptive control mechanism. To examine the possibility that internal spatial representations undergo adaptation, we had participants perform a task that interleaved a perceptual discrimination task and a spatial working memory task. Perceptual discrimination trials (85% of trials) presented an initial peripheral cue to exogenously capture attention, immediately followed by a displaced target stimulus. This sequence of events served to repeatedly induce a covert attentional allocation error. Interleaved spatial working memory trials (15% of trials) presented a stimulus at a pseudorandom peripheral location followed by a delay interval. On half of the working memory trials, the stimulus was surreptitiously presented at the same location as the initial attentional cue. We found that as attentional errors accumulated over the course of the experiment, participants’ spatial recall shifted to counteract the attentional error. The magnitude of this shift was proportional to the number of induced errors. Recall performance recovered rapidly following the offset of error trials. Multiple control experiments ruled out alternative explanations for these results, such as oculomotor confounds and attentional biases unrelated to error. These findings indicate that the computational mechanisms governing the adaptation of motor commands appear to similarly serve to adjust and calibrate spatial cognition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available at https://osf.io/egskw/.
Code availability
The analysis code supporting the findings of this study is available on GitHub at https://github.com/brissend/WM_adapt.
Change history
18 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41562-025-02170-0
References
Shadmehr, R., Smith, M. A. & Krakauer, J. W. Error correction, sensory prediction, and adaptation in motor control. Neuroscience 33, 89–108 (2010).
McLaughlin, S. C. Parametric adjustment in saccadic eye movements. Percept. Psychophys. 2, 359–362 (1967).
Deubel, H., Wolf, W. & Hauske, G. Adaptive gain control of saccadic eye movements. Hum. Neurobiol. 5, 245–253 (1986).
Frens, M. A. & van Opstal, A. J. Transfer of short-term adaptation in human saccadic eye movements. Exp. Brain Res. 100, 293–306 (1994).
Straube, A., Fuchs, A. F., Usher, S. & Robinson, F. R. Characteristics of saccadic gain adaptation in rhesus macaques. J. Neurophysiol. 77, 874–895 (1997).
Hopp, J. J. & Fuchs, A. F. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog. Neurobiol. 72, 27–53 (2004).
Watanabe, S., Ogino, S., Nakamura, T. & Koizuka, I. Saccadic adaptation in the horizontal and vertical directions in normal subjects. Auris Nasus Larynx 30, 41–45 (2003).
Mazzoni, P. & Krakauer, J. W. An implicit plan overrides an explicit strategy during visuomotor adaptation. J. Neurosci. 26, 3642–3645 (2006).
Taylor, J. A., Krakauer, J. W. & Ivry, R. B. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci. 34, 3023–3032 (2014).
McDougle, S. D., Bond, K. M. & Taylor, J. A. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J. Neurosci. 35, 9568–9579 (2015).
van der Kooij, K., Wijdenes, L. O., Rigterink, T., Overvliet, K. E. & Smeets, J. B. J. Reward abundance interferes with error-based learning in a visuomotor adaptation task. PLoS ONE 13, 1–12 (2018).
Albert, S. T. et al. An implicit memory of errors limits human sensorimotor adaptation. Nat. Hum. Behav. 5, 920–934 (2021).
Awh, E., Vogel, E. K. & Oh, S.-H. Interactions between attention and working memory. Neuroscience 139, 201–208 (2006).
Schmidt, B. K., Vogel, E. K., Woodman, G. F. & Luck, S. J. Voluntary and automatic attentional control of visual working memory. Percept. Psychophys. 64, 754–763 (2002).
Cowan, N. & Morey, C. C. Visual working memory depends on attentional filtering. Trends Cogn. Sci. 10, 139–141 (2006).
Chun, M. M. & Turk-Browne, N. B. Interactions between attention and memory. Curr. Opin. Neurobiol. 17, 177–184 (2007).
McNab, F. & Klingberg, T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 11, 103–107 (2008).
Kiyonaga, A. & Egner, T. Working memory as internal attention: toward an integrative account of internal and external selection processes. Psychon. Bull. Rev. 20, 228–242 (2013).
Fougnie, D. & Marois, R. Attentive tracking disrupts feature binding in visual working memory. Vis. Cogn. 17, 48–66 (2009).
Pélisson, D., Alahyane, N., Panouillères, M. & Tilikete, C. Sensorimotor adaptation of saccadic eye movements. Neurosci. Biobehav. Rev. 34, 1103–1120 (2010).
Noto, C. T., Watanabe, S. & Fuchs, A. F. Characteristics of simian adaptation fields produced by behavioral changes in saccade size and direction. J. Neurophysiol. 81, 2798–2813 (1999).
Smith, M. A., Ghazizadeh, A. & Shadmehr, R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 4, 1035–1043 (2006).
Maljkovic, V. & Nakayama, K. Priming of pop-out: II. The role of position. Percept. Psychophys. 58, 977–991 (1996).
Ede, Fvan Visual working memory and action: functional links and bi-directional influences. Vis. Cogn. 28, 401–413 (2020).
Seidler, R. D., Bo, J. & Anguera, J. A. Neurocognitive contributions to motor skill learning: the role of working memory. J. Mot. Behav. 44, 445–453 (2012).
Anguera, J. A., Reuter-Lorenz, P. A., Willingham, D. T. & Seidler, R. D. Contributions of spatial working memory to visuomotor learning. J. Cogn. Neurosci. 22, 1917–1930 (2010).
Anguera, J. A., Reuter-Lorenz, P. A., Willingham, D. T. & Seidler, R. D. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J. Cogn. Neurosci. 23, 11–25 (2011).
Benson, B. L., Anguera, J. A. & Seidler, R. D. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J. Neurophysiol. 105, 2843–2851 (2011).
Anguera, J. A. et al. The effects of working memory resource depletion and training on sensorimotor adaptation. Behav. Brain Res. 228, 107–115 (2012).
Taylor, J. A. & Ivry, R. B. Flexible cognitive strategies during motor learning. PLoS Comput. Biol. 7, 1–13 (2011).
McDougle, S. D. & Taylor, J. A. Dissociable cognitive strategies for sensorimotor learning. Nat. Commun. 10, 1–13 (2019).
Wolpert, D. M., Miall, R. C. & Kawato, M. Internal models in the cerebellum. Trends Cogn. Sci. 2, 338–347 (1998).
Bastian, A. J. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr. Opin. Neurobiol. 16, 645–649 (2006).
Tzvi, E., Loens, S. & Donchin, O. Mini-review: the role of the cerebellum in visuomotor adaptation. Cerebellum 21, 306–313 (2022).
Miall, R. C. & Wolpert, D. M. Forward models for physiological motor control. Neural Netw. 9, 1265–1279 (1996).
Imamizu, H. et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403, 192–195 (2000).
Tseng, Y., Diedrichsen, J., Krakauer, J. W., Shadmehr, R. & Bastian, A. J. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol. 98, 54–62 (2007).
Therrien, A. S. & Bastian, A. J. Cerebellar damage impairs internal predictions for sensory and motor function. Curr. Opin. Neurobiol. 33, 127–133 (2015).
Schlerf, J., Ivry, R. B. & Diedrichsen, J. Encoding of sensory prediction errors in the human cerebellum. J. Neurosci. 32, 4913–4922 (2012).
Tanaka, H., Ishikawa, T., Lee, J. & Kakei, S. The cerebro-cerebellum as a locus of forward model: a review. Front. Syst. Neurosci. 14, 1–16 (2020).
Raymond, J. L. & Medina, J. F. Computational principles of supervised learning in the cerebellum. Annu. Rev. Neurosci. 41, 233–253 (2018).
Allen, G., Buxton, R. B., Wong, E. C. & Courchesne, E. Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943 (1997).
Chen, S. H. A. & Desmond, J. E. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24, 332–338 (2005).
Chen, S. H. A. & Desmond, J. E. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia 43, 1227–1237 (2005).
Kirschen, M. P., Chen, S. H. A., Schraedley-Desmond, P. & Desmond, J. E. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage 24, 462–472 (2005).
Stoodley, C. J., Valera, E. M. & Schmahmann, J. D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570 (2012).
Brissenden, J. A., Levin, E. J., Osher, D. E., Halko, M. A. & Somers, D. C. Functional evidence for a cerebellar node of the dorsal attention network. J. Neurosci. 36, 6083–6096 (2016).
Brissenden, J. A. et al. Topographic cortico-cerebellar networks revealed by visual attention and working memory. Curr. Biol. 28, 3364–3372.e5 (2018).
Brissenden, J. A., Tobyne, S. M., Halko, M. A. & Somers, D. C. Stimulus-specific visual working memory representations in human cerebellar lobule VIIb/VIIIa. J. Neurosci. 41, 1033–1045 (2021).
Sokolov, A. A., Miall, R. C. & Ivry, R. B. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn. Sci. 21, 313–332 (2017).
Guell, X., Schmahmann, J. D., Gabrieli, J. D. & Ghosh, S. S. Functional gradients of the cerebellum. eLife 7, 1–22 (2018).
King, M., Hernandez-Castillo, C. R., Poldrack, R. A., Ivry, R. B. & Diedrichsen, J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 22, 1371–1378 (2019).
Hayter, A. L., Langdon, D. W. & Ramnani, N. Cerebellar contributions to working memory. Neuroimage 36, 943–954 (2007).
Itō, M. The Cerebellum and Neural Control (Raven Press, 1984).
Ramnani, N. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci. 7, 511–522 (2006).
Schmahmann, J. D. The role of the cerebellum in affect and psychosis. J. Neurolinguistics 13, 189–214 (2000).
Ito, M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 9, 304–313 (2008).
Herzfeld, D. J., Kojima, Y., Soetedjo, R. & Shadmehr, R. Encoding of error and learning to correct that error by the Purkinje cells of the cerebellum. Nat. Neurosci. 21, 736–743 (2018).
Woodman, G. F. & Vogel, E. K. Fractionating working memory. Psychol. Sci. 16, 106–113 (2004).
Todd, J. J., Han, S. W., Harrison, S. & Marois, R. The neural correlates of visual working memory encoding: a time-resolved fMRI study. Neuropsychologia 49, 1527–1536 (2011).
Ye, C. et al. A two-phase model of resource allocation in visual working memory. J. Exp. Psych. Learn. Mem. Cogn. 43, 1557–1566 (2017).
Carrasco, M. Visual attention: the past 25 years. Vision Res. 51, 1484–1525 (2011).
McFadden, S. A., Khan, A. & Wallman, J. Gain adaptation of exogenous shifts of visual attention. Vision Res. 42, 2709–2726 (2002).
Hikosaka, O., Miyauchi, S. & Shimojo, S. Visual attention revealed by an illusion of motion. Neurosci. Res. 18, 11–18 (1993).
Downing, P. E. & Treisman, A. M. The line–motion illusion: attention or impletion? J. Exp. Psychol. Hum. Percept. Perform. 23, 768–779 (1997).
Tse, P., Cavanagh, P. & Nakayama, K. in High-level Motion Processing: Computational, Neurobiological and Pychophysical Perspectives (ed. Watanabe, T.) 244–261 (MIT Press, 1998).
Schmidt, W. C. Endogenous attention and illusory line motion reexamined. J. Exp. Psychol. Hum. Percept. Perform. 26, 980–996 (2000).
Christie, J. & Klein, R. M. Does attention cause illusory line motion? Percept. Psychophys. 67, 1032–1043 (2005).
Ono, F., Yamada, Y., Takahashi, K., Sasaki, K. & Ariga, A. Backward illusory line motion: visual motion perception can be influenced by retrospective stimulation. J. Vis. 23, 1–11 (2023).
Bahcall, D. O. & Kowler, E. Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature 400, 864–866 (1999).
Hernandez, T. D., Levitan, C. A., Banks, M. S. & Schor, C. M. How does saccade adaptation affect visual perception? J Vis. 8, 1–16 (2008).
Schnier, F., Zimmermann, E. & Lappe, M. Adaptation and mislocalization fields for saccadic outward adaptation in humans. J. Eye Mov. Res. 3, 1–18 (2010).
Cheviet, A. et al. Cerebellar signals drive motor adjustments and visual perceptual changes during forward and backward adaptation of reactive saccades. Cereb. Cortex 32, 3896–3916 (2022).
Awater, H., Burr, D., Lappe, M., Morrone, M. C. & Goldberg, M. E. Effect of saccadic adaptation on localization of visual targets. J. Neurophysiol. 93, 3605–3614 (2005).
Collins, T., Doré-Mazars, K. & Lappe, M. Motor space structures perceptual space: evidence from human saccadic adaptation. Brain Res. 1172, 32–39 (2007).
Georg, K. & Lappe, M. Effects of saccadic adaptation on visual localization before and during saccades. Exp. Brain Res. 192, 9–23 (2009).
Sperling, G. The information available in brief visual presentations. Psychol. Monogr. Gen. Appl. 74, 1–29 (1960).
Loftus, G. R., Duncan, J. & Gehrig, P. On the time course of perceptual information that results from a brief visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 18, 530–549 (1992).
Lu, Z.-L., Neuse, J., Madigan, S. & Dosher, B. A. Fast decay of iconic memory in observers with mild cognitive impairments. Proc. Natl Acad. Sci. USA 102, 1797–1802 (2005).
Pratte, M. S. Iconic memories die a sudden death. Psychol. Sci. 29, 877–887 (2017).
Teeuwen, R. R. M., Wacongne, C., Schnabel, U. H., Self, M. W. & Roelfsema, P. R. A neuronal basis of iconic memory in macaque primary visual cortex. Curr. Biol. 31, 5401–5414.e4 (2021).
Tomić, I. & Bays, P. M. A dynamic neural resource model bridges sensory and working memory. eLife 12, 1–38 (2024).
Averbach, E. & Coriell, A. S. Short-term memory in vision. Bell Syst. Tech. J. 40, 309–328 (1961).
Mewhort, D. J., Merikle, P. M. & Bryden, M. P. On the transfer from iconic to short-term memory. J. Exp. Psychol. 81, 89–94 (1969).
Phillips, W. A. On the distinction between sensory storage and short-term visual memory. Percept. Psychophys. 16, 283–290 (1974).
Coltheart, M. Iconic memory and visible persistence. Percept. Psychophys. 27, 183–228 (1980).
Gegenfurtner, K. R. & Sperling, G. Information transfer in iconic memory experiments. J. Exp. Psychol. Hum. Percept. Perform. 19, 845–866 (1993).
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C. & Yeo, B. T. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815 (2013).
Manohar, S. G. et al. Reward pays the cost of noise reduction in motor and cognitive control. Curr. Biol. 25, 1707–1716 (2015).
Egger, S. W., Remington, E. D., Chang, C.-J. & Jazayeri, M. Internal models of sensorimotor integration regulate cortical dynamics. Nat. Neurosci. 22, 1871–1882 (2019).
Ritz, H., Frömer, R. & Shenhav, A. Bridging motor and cognitive control: it’s about time! Trends Cogn. Sci. 24, 6–8 (2020).
Peirce, J. et al. PsychoPy2: experiments in behavior made easy. Behav. Res. Methods 51, 195–203 (2019).
Peirce, J. W. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 162, 8–13 (2007).
Peirce, J. W. Generating stimuli for neuroscience using PsychoPy. Front. Neuroinform. 2, 1–8 (2008).
Morey, R. & Rouder, J. N. BayesFactor: computation of Bayes factors for common designs. The Comprehensive R Archive Network https://cran.radicaldevelop.com/web/packages/BayesFactor/BayesFactor.pdf (2023).
Jeffreys, H. An invariant form for the prior probability in estimation problems. Proc. R. Soc. Lond. A 186, 453–461 (1946).
Zellner, A. & Siow, A. Posterior odds ratios for selected regression hypotheses. Trab. Estad. Y Investig. Oper. 31, 585–603 (1980).
Rouder, J. N., Morey, R. D., Speckman, P. L. & Province, J. M. Default Bayes factors for ANOVA designs. J. Math. Psychol. 56, 356–374 (2012).
Robinson, F. R., Soetedjo, R. & Noto, C. Distinct short-term and long-term adaptation to reduce saccade size in monkey. J. Neurophysiol. 96, 1030–1041 (2006).
Rotella, M. F. et al. Learning and generalization in an isometric visuomotor task. J. Neurophysiol. 113, 1873–1884 (2015).
Stan Development Team. RStan: The R Interface to Stan (2023). https://mc-stan.org/
Bürkner, P.-C. Advanced Bayesian multilevel modeling with the R package brms. R Journal 10, 395–411 (2018).
Bürkner, P.-C. brms: an R package for bayesian multilevel models using stan. J. Stat. Softw. 80, 1–28 (2017).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
Vehtari, A. et al. loo: Efficient leave-one-out cross-validation and WAIC for bayesian models. R package version 2.6.0. https://cran.r-project.org/web/packages/loo/loo.pdf (2023).
Sivula, T., Magnusson, M., Matamoros, A. A. & Vehtari, A. Uncertainty in Bayesian leave-one-out cross-validation based model comparison. Preprint at https://doi.org/10.48550/arXiv.2008.10296 (2020).
Geller, J., Winn, M. B., Mahr, T. & Mirman, D. GazeR: a package for processing gaze position and pupil size data. Behav. Res. Methods 52, 2232–2255 (2020).
Acknowledgements
We thank T. Adkins and Q. Nguyen for helpful comments and discussion. This work was supported by National Institutes of Health grant no. F32MH124268 (J.A.B.). The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
J.A.B. and T.G.L. conceived the study. J.A.B. and Y.Y. collected the data. J.A.B. carried out data analysis and wrote the original draft of the paper. All authors reviewed the paper and provided critical revisions. T.G.L. and M.V. provided resources and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Edward Ester and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brissenden, J.A., Yin, Y., Vesia, M. et al. Errors of attention adaptively warp spatial cognition. Nat Hum Behav 9, 769–780 (2025). https://doi.org/10.1038/s41562-025-02109-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41562-025-02109-5