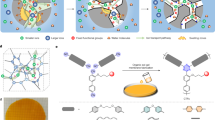
Abstract
Nanofluidics have led to the discovery of unconventional properties for water and ion transport at the nanoscale, but key challenges remain in their large-scale implementation. Here we report an osmotic resonance across macroscopic composite membranes made by the assembly of microporous and mesoporous layers, taking root from the rectified osmotic transport in nanopores. This osmotic diode induces ionic sieving and continuous fast macroscopic electro-osmotic transport. This is the basis for a versatile approach for water purification, by which fresh water is driven across a composite material under an a.c. electric field. Water flow is driven within the mesoporous layer, while selectivity is achieved within the microporous layer. The maximal rectified, diode-like water flow is found to be in the hertz range. Building on analytical predictions, we show that a conversion factor of up to ~15 equivalent bars per applied volt can be reached using appropriate materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. These data are also available via Zenodo at https://doi.org/10.5281/zenodo.15277891 (ref. 44).
References
Bocquet, L. Nanofluidics coming of age. Nat. Mater. 19, 254–256 (2020).
Aluru, N. R. et al. Fluids and electrolytes under confinement in single-digit nanopores. Chem. Rev. 123, 2737–2831 (2023).
Deshmukh, A. et al. Membrane distillation at the water-energy nexus: limits, opportunities, and challenges. Energy Environ. Sci. 11, 1177–1196 (2018).
Li, Z., Siddiqi, A., Anadon, L. D. & Narayanamurti, V. Towards sustainability in water-energy nexus: ocean energy for seawater desalination. Renew. Sustain. Energy Rev. 82, 3833–3847 (2018).
Esfandiar, A. et al. Size effect in ion transport through angstrom-scale slits. Science 358, 511–513 (2017).
Prakash, S., Pinti, M. & Bhushan, B. Theory, fabrication and applications of microfluidic and nanofluidic biosensors. Phil. Trans. R. Soc. A 370, 2269–2303 (2012).
Piruska, A., Gong, M., Sweedler, J. V. & Bohn, P. W. Nanofluidics in chemical analysis. Chem. Soc. Rev. 39, 1060–1072 (2010).
Ries, L. et al. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. 18, 1112–1117 (2019).
Li, X. et al. Nature gives the best solution for desalination: aquaporin-based hollow fiber composite membrane with superior performance. J. Membr. Sci. 494, 68–77 (2015).
Törnroth-Horsefield, S. et al. Structural mechanism of plant aquaporin gating. Nature 439, 688–694 (2006).
Emmerich, T. et al. Enhanced nanofluidic transport in activated carbon nanoconduits. Nat. Mater. 21, 696–702 (2022).
Robin, P. et al. Long-term memory and synapse-like dynamics in two-dimensional nanofluidic channels. Science 379, 161–167 (2023).
Xu, Y. Nanofluidics: a new arena for materials science. Adv. Mater. 30, 1702419 (2018).
Herman, A., Ager, J. W., Ardo, S. & Segev, G. Ratchet-based ion pumps for selective ion separations. PRX Energy 2, 023001 (2023).
Huang, X., Kong, X., Wen, L. & Jiang, L. Bioinspired ionic diodes: from unipolar to bipolar. Adv. Funct. Mater. 28, 1801079 (2018).
Karnik, R., Duan, C., Castelino, K., Daiguji, H. & Majumdar, A. Rectification of ionic current in a nanofluidic diode. Nano Lett. 7, 547–551 (2007).
Cheng, L.-J. & Guo, L. J. Nanofluidic diodes. Chem. Soc. Rev. 39, 923–938 (2010).
Poggioli, A. R., Siria, A. & Bocquet, L. Beyond the tradeoff: dynamic selectivity in ionic transport and current rectification. J. Phys. Chem. B 123, 1171–1185 (2019).
Montes de Oca, J. M., Dhanasekaran, J., Córdoba, A., Darling, S. B. & de Pablo, J. J. Ionic transport in electrostatic Janus membranes. An explicit solvent molecular dynamic simulation. ACS Nano 16, 3768–3775 (2022).
Picallo, C. B., Gravelle, S., Joly, L., Charlaix, E. & Bocquet, L. Nanofluidic osmotic diodes: theory and molecular dynamics simulations. Phys. Rev. Lett. 111, 244501 (2013).
Ratschow, A. D., Pandey, D., Liebchen, B., Bhattacharyya, S. & Hardt, S. Resonant nanopumps: ac gate voltages in conical nanopores induce directed electrolyte flow. Phys. Rev. Lett. 129, 264501 (2022).
Laohakunakorn, N. et al. A Landau–Squire nanojet. Nano Lett. 13, 5141–5146 (2013).
Wu, X., Ramiah Rajasekaran, P. & Martin, C. R. An alternating current electroosmotic pump based on conical nanopore membranes. ACS Nano 10, 4637–4643 (2016).
Alizadeh, A., Hsu, W., Wang, M. & Daiguji, H. Electroosmotic flow: from microfluidics to nanofluidics. Electrophoresis 42, 834–868 (2021).
Wen, Q. et al. Electric‐field‐induced ionic sieving at planar graphene oxide heterojunctions for miniaturized water desalination. Adv. Mater. 32, 1903954 (2020).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Mi, B. Scaling up nanoporous graphene membranes. Science 364, 1033–1034 (2019).
Lee, B., Wang, L., Wang, Z., Cooper, N. J. & Elimelech, M. Directing the research agenda on water and energy technologies with process and economic analysis. Energy Environ. Sci. 16, 714–722 (2023).
Marbach, S. & Bocquet, L. Osmosis, from molecular insights to large-scale applications. Chem. Soc. Rev. 48, 3102–3144 (2019).
Devasenathipathy, S. & Santiago, J. G. in Microscale Diagnostic Techniques (ed. Breuer, K. S.) 113–154 (Springer, 2005); https://doi.org/10.1007/3-540-26449-3_3
Huisman, I. Electroviscous effects, streaming potential, and zeta potential in polycarbonate track-etched membranes. J. Membr. Sci. 178, 79–92 (2000).
Van Der Heyden, F. H. J., Stein, D. & Dekker, C. Streaming currents in a single nanofluidic channel. Phys. Rev. Lett. 95, 116104 (2005).
Jia, F. et al. Advances in graphene oxide membranes for water treatment. Nano Res. 15, 6636–6654 (2022).
Yang, Q. et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 16, 1198–1202 (2017).
Béguin, F., Presser, V., Balducci, A. & Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 26, 2219–2251 (2014).
Suss, M. E. & Presser, V. Water desalination with energy storage electrode materials. Joule 2, 10–15 (2018).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Geise, G. M., Park, H. B., Sagle, A. C., Freeman, B. D. & McGrath, J. E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 369, 130–138 (2011).
Ober, P. et al. Liquid flow reversibly creates a macroscopic surface charge gradient. Nat. Commun. 12, 4102 (2021).
Werkhoven, B. L., Everts, J. C., Samin, S. & van Roij, R. Flow-induced surface charge heterogeneity in electrokinetics due to Stern-layer conductance coupled to reaction kinetics. Phys. Rev. Lett. 120, 264502 (2018).
Biesheuvel, P. M., Porada, S., Elimelech, M. & Dykstra, J. E. Tutorial review of reverse osmosis and electrodialysis. J. Membr. Sci. 647, 120221 (2022).
Wang, L. et al. Water transport in reverse osmosis membranes is governed by pore flow, not a solution-diffusion mechanism. Sci. Adv. 9, eadf8488 (2023).
Biesheuvel, P. M., Rutten, S. B., Ryzhkov, I. I., Porada, S. & Elimelech, M. Theory for salt transport in charged reverse osmosis membranes: novel analytical equations for desalination performance and experimental validation. Desalination 557, 116580 (2023).
Abdelghani-Idrissi, S. Dataset for the manuscript entitled ‘Resonant osmotic diodes for voltage-induced water filtration across composite membranes’. Zenodo https://doi.org/10.5281/ZENODO.15277891 (2025).
Acknowledgements
We thank V. Pichon, P. Simon and P.-L. Tabernat for fruitful discussions of water analysis techniques and capacitive electrode behaviours; B. Bresson for technical assistance regarding SEM imaging of membranes; B. Cinquin and the technological platform of the Institut Pierre-Gilles de Gennes (IPGG) for the availability of UV–visible absorbance equipment; and A. Grimaud for the availability of XRD equipment. L.B. acknowledges funding from the EU Horizon 2020 Framework Programme and European Research Council (ERC) Synergy Grant, agreement no. 101071937-n-AQUA. J.P.-C. acknowledges funding from Community of Madrid programme no. 2020-T2/IND-20074.
Author information
Authors and Affiliations
Contributions
L.B. conceived the project. L.R., Z.P., P.S. and J.P.-C. developed the membranes, and S.A.-I. developed the experimental set-ups with input from L.B. and A.S. and S.A.-I. performed the experiments with inputs from Z.P. and P.S. G.M. performed the numerical simulations. L.B. developed the theoretical description with input from S.A.-I., and S.A.-I. and L.B. wrote the paper, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
CNRS has filed patent applications on the technologies described in this manuscript with some co-authors listed as inventors (WO2021156393A1, EP4410407A1). Based on these patents, the start-up Ilion Water Technologies was founded in February 2025, with L.R., Z.P., P.S. and L.B. as cofounders.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Tables 1–5, captions for Videos 1–4, text and refs. 44–47.
Supplementary Video 1
Typical a.c. EO filtration of a rhodamine 6G solution using a PC + MoS2 membrane. The experiment is performed with a 10 V amplitude at resonant frequency. The speed is ×64. The initial rhodamine concentration of the feed syringe is 0.055 mM, and the final measured concentration in the feed side of the cell is ~10 times the initial feed concentration, as expected by the mass balance. On the permeate side, we do not report any increase of rhodamine concentration.
Supplementary Video 2
Typical a.c. EO experiment at 10 V amplitude and a low frequency (2.5 mHz) using a PC (100 nm) + GO membrane. The asymmetry of the EO flow rate is clearly visible. The speed is ×64.
Supplementary Video 3
Typical a.c. EO experiment at 10 V amplitude and a low frequency (2.5 mHz) using a pristine PC (100 nm) membrane. The EO flow rate is clearly symmetric and not rectified in this situation. The speed is ×64.
Supplementary Video 4
Typical a.c. EO filtration of a rhodamine 6G solution using a PC (200 nm) + GO membrane. The experiment is performed with a 5 V amplitude at 0.1 Hz. The filtered volume is 10 ml. The speed is ×480. The initial rhodamine concentration of the feed syringe is 0.05 mM in a 10 ml water volume with 10−3 M NaCl. On the permeate side, we do not report any increase of rhodamine concentration.
Source data
Source Data Fig. 2
Source data for results in Fig. 2.
Source Data Fig. 3
Source data for results in Fig. 3.
Source Data Fig. 4
Source data for results in Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelghani-Idrissi, S., Ries, L., Monet, G. et al. Resonant osmotic diodes for voltage-induced water filtration across composite membranes. Nat. Mater. 24, 1109–1115 (2025). https://doi.org/10.1038/s41563-025-02257-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02257-z