Abstract
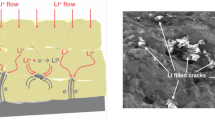
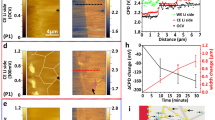
We report a method for promoting electrochemical stability in garnet Li6.4La3Zr1.4Ta0.6O12 solid-state electrolyte based on a composite two-phase oxide–oxide microstructure. Grain boundary precipitation of the controlled distribution of amorphous zirconium oxide microparticles is achieved through the addition of reactive tantalum carbide. During ambient-atmosphere sintering, the carbide decomposes through an in situ reaction, the ‘extra’ Ta substituting for Zr within the Li6.4La3Zr1.4Ta0.6O12 lattice. Density functional theory (DFT) calculations identify a thermodynamically favourable reaction path and show how substituting Ta5+ at Zr4+ sites affects the crystal structure as well as bulk ionic and electronic conductivities. Quantitative stereology highlights that zirconia also acts as a sintering aid, reducing compact porosity. Cryogenic focused-ion-beam scanning electron microscopy and fractography analysis of cycled solid-state electrolytes illustrates that near-universally observed intergranular Li-metal dendrite propagation is suppressed by the two-phase microstructure, favouring transgranular dendrites instead. Importantly, DFT demonstrates that compared with the Li6.4La3Zr1.4Ta0.6O12 surface, the zirconium oxide surface per se is less electronically conductive and does not trap excess electrons to reduce Li ions. This is a key reason for the substantial improvement in the electrochemical properties over the single-phase baseline.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Samson, A. J., Hofstetter, K., Bag, S. & Thangadurai, V. A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ. Sci. 12, 2957–2975 (2019).
Bachman, J. C. et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016).
Thangadurai, V., Narayanan, S. & Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem. Soc. Rev. 43, 4714–4727 (2014).
Allen, J. L., Wolfenstine, J., Rangasamy, E. & Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 206, 315–319 (2012).
David, I. N., Thompson, T., Wolfenstine, J., Allen, J. L. & Sakamoto, J. Microstructure and Li-ion conductivity of hot-pressed cubic Li7La3Zr2O12. J. Am. Ceram. Soc. 98, 1209–1214 (2015).
Baek, S. W., Lee, J. M., Kim, T. Y., Song, M. S. & Park, Y. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 249, 197–206 (2014).
Wu, Y. et al. Rapid processing of uniform, thin, robust, and large-area garnet solid electrolyte by atmospheric plasma spraying. Adv. Energy Mater. 13, 2300809 (2023).
Alexander, G. V., Shi, C., O’Neill, J. & Wachsman, E. D. Extreme lithium-metal cycling enabled by a mixed ion- and electron-conducting garnet three-dimensional architecture. Nat. Mater. 22, 1136–1143 (2023).
Pfenninger, R., Struzik, M., Garbayo, I., Stilp, E. & Rupp, J. L. M. A low ride on processing temperature for fast lithium conduction in garnet solid-state battery films. Nat. Energy 4, 475–483 (2019).
Albertus, P., Babinec, S., Litzelman, S. & Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018).
Raj, V., Aetukuri, N. P. B. & Nanda, J. Solid state lithium metal batteries—issues and challenges at the lithium-solid electrolyte interface. Curr. Opin. Solid State Mater. Sci. 26, 100999 (2022).
Wang, Y. et al. Stable anode-free all-solid-state lithium battery through tuned metal wetting on the copper current collector. Adv. Mater. 35, 2206762 (2023).
Kasemchainan, J. et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019).
Lewis, J. A. et al. Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography. Nat. Mater. 20, 503–510 (2021).
Cheng, L. et al. Effect of surface microstructure on electrochemical performance of garnet solid electrolytes. ACS Appl. Mater. Interfaces 7, 2073–2081 (2015).
Raj, V. et al. Dendrite growth—microstructure—stress—interrelations in garnet solid-state electrolyte. Adv. Energy Mater. 14, 2303062 (2024).
Tian, H.-K., Liu, Z., Ji, Y., Chen, L.-Q. & Qi, Y. Interfacial electronic properties dictate Li dendrite growth in solid electrolytes. Chem. Mater. 31, 7351–7359 (2019).
Raj, V. et al. Direct correlation between void formation and lithium dendrite growth in solid-state electrolytes with interlayers. Nat. Mater. 21, 1050–1056 (2022).
Klinsmann, M., Hildebrand, F. E., Ganser, M. & McMeeking, R. M. Dendritic cracking in solid electrolytes driven by lithium insertion. J. Power Sources 442, 227226 (2019).
Liu, X. et al. Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021).
Feng, M., Pan, J. & Qi, Y. Impact of electronic properties of grain boundaries on the solid electrolyte interphases (SEIs) in Li-ion batteries. J. Phys. Chem. C 125, 15821–15829 (2021).
Albertus, P. et al. Challenges for and pathways toward Li-metal-based all-solid-state batteries. ACS Energy Lett. 6, 1399–1404 (2021).
Kalnaus, S., Dudney, N. J., Westover, A. S., Herbert, E. & Hackney, S. Solid-state batteries: the critical role of mechanics. Science 381, eabg5998 (2023).
Barai, P., Higa, K., Ngo, A. T., Curtiss, L. A. & Srinivasan, V. Mechanical stress induced current focusing and fracture in grain boundaries. J. Electrochem. Soc. 166, A1752 (2019).
Vishnugopi, B. S. et al. Asymmetric contact loss dynamics during plating and stripping in solid-state batteries. Adv. Energy Mater. 13, 2203671 (2023).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Xu, Y. et al. Improved Li-ion conduction and (electro)chemical stability at garnet-polymer interface through metal-nitrogen bonding. Adv. Energy Mater. 13, 2204377 (2023).
Tsai, C. L. et al. Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Appl. Mater. Interfaces 8, 10617–10626 (2016).
Fu, K. et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc. Natl Acad. Sci. USA 113, 7094–7099 (2016).
Lee, C. et al. Stack pressure measurements to probe the evolution of the lithium–solid-state electrolyte interface. ACS Energy Lett. 6, 3261–3269 (2021).
Campanella, D. et al. Gram-scale carbothermic control of LLZO garnet solid electrolyte particle size. Chem. Eng. J. 457, 141349 (2023).
Li, Y., Han, J. T., Wang, C. A., Xie, H. & Goodenough, J. B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 22, 15357–15361 (2012).
Hannink, R. H. J., Kelly, P. M. & Muddle, B. C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 83, 461–487 (2000).
Wang, J. & Stevens, R. Zirconia-toughened alumina (ZTA) ceramics. J. Mater. Sci. 24, 3421–3440 (1989).
Bach, J. P. & Thevenot, F. Fabrication and characterization of zirconia-toughened alumina obtained by inorganic and organic precursors. J. Mater. Sci. 24, 2711–2721 (1989).
He, X., Zhu, Y. & Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Klimpel, M., Zhang, H., Kovalenko, M. V. & Kravchyk, K. V. Standardizing critical current density measurements in lithium garnets. Commun. Chem. 6, 192 (2023).
Jelitto, H. & Schneider, G. A. A geometric model for the fracture toughness of porous materials. Acta Mater. 151, 443–453 (2018).
Ashby, M. F. & Gibson, L. J. Cellular Solids: Structure and Properties (Cambridge Univ. Press, 1997).
Raj, R. & Wolfenstine, J. Current limit diagrams for dendrite formation in solid-state electrolytes for Li-ion batteries. J. Power Sources 343, 119–126 (2017).
Newman, J. C. Stress-intensity factors and crack-opening displacements for round compact specimens. Int. J. Fract. 17, 567–578 (1981).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223–16233 (1994).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Tian, H.-K., Xu, B. & Qi, Y. Computational study of lithium nucleation tendency in Li7La3Zr2O12 (LLZO) and rational design of interlayer materials to prevent lithium dendrites. J. Power Sources 392, 79–86 (2018).
Acknowledgements
Y.W., H.F., P.J., M.F. and Y.Q. were supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE) through the Advanced Battery Materials Research Program (Battery500 Consortium, contract number 674882). V.R., K.G.N., B.S.V., P.P.M. and D.M. were supported by the Mechano-Chemical Understanding of Solid Ion Conductors, an Energy Frontier Research Center funded by the US DOE, Office of Science, Office of Basic Energy Science, contract number DE-SC0023438. S.K.’s research at Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the US DOE under contract number DE-AC05-00OR22725, was supported by the US DOE’s Office of Energy Efficiency and Renewable Energy for the Vehicle Technologies Office’s Advanced Battery Materials Research Program. H.F. acknowledges the Office of Advanced Research Computing (OARC) at Rutgers, The State University of New Jersey for providing access to the Amarel cluster and associated research computing resources that have contributed to the results reported here. H.F. and P.J. acknowledge resources from the National Renewable Energy Laboratory High-Performance Computing Facilities (with Kestrel and Swift computing systems). The acquisition of the VersaProbe-IV XPS was supported by the National Science Foundation Major Research Instrumentation program (grant number 2117623). This work was performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility operated by the US DOE’s Office of Science. Los Alamos National Laboratory, an affirmative action equal opportunity employer, is managed by Triad National Security, LLC, for the US DOE’s National Nuclear Security Administration (NNSA), under contract number 89233218CNA000001. This research used the hard X-ray nanoprobe beamline at 3-ID of the National Synchrotron Light Source II, US DOE, Office of Science User Facility, operated for the DOE’s Office of Science by Brookhaven National Laboratory under contract number DESC0012704. This work was supported by The Welch Foundation (F-2206). Sandia National Laboratories is a multi-mission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC (NTESS), a wholly owned subsidiary of Honeywell International Inc., for DOE/NNSA under contract number DE-NA0003525. This written work is authored by an employee of NTESS. The employee, not NTESS, owns the right, title and interest in and to the written work and is responsible for its contents. Any subjective views or opinions that might be expressed in the written work do not necessarily represent the views of the US government. The publisher acknowledges that the US government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this written work or allow others to do so, for US government purposes. The DOE will provide public access to results of federally sponsored research in accordance with the DOE Public Access Plan.
Author information
Authors and Affiliations
Contributions
V.R., Y.W. and D.M. conceived the idea and designed the experiments. V.R., Y.W. and A.S.M. performed the synthesis, materials characterization and electrochemical measurements. S.D., X.H. and Y.L. carried out the synchrotron experiments and related data analysis. M.F., K.G.N., B.S.V., H.F., P.J., P.P.M. and Y.Q. carried out the computational simulations. N.B.S., M.S., A.M.H. and J.D.M. helped with the electrochemical measurements. M.J. and B.L.B. performed the mechanical property measurements. S.K. and J.W. participated in the discussion of data analysis. V.R., Y.W. and D.M. wrote the first draft of the paper. All authors discussed the results and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
V.R. and D.M. are inventors on US patent applications (PCT/US2024/029976) related to reactive synthesis methods. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–33, Tables 1–9, and Notes 1 and 2.
Supplementary Video 1
SEM video displaying lithium nucleation on LLZTO upon electron exposure.
Supplementary Data 1
A text file containing the computational codes and data.
Supplementary Data 2
DFT calculation for the optimized structures.
Supplementary Data 3
DFT calculation for the optimized structures.
Supplementary Data 4
DFT calculation for the optimized structures.
Supplementary Data 5
DFT calculation for the optimized structures.
Supplementary Data 6
DFT calculation for the optimized structures.
Supplementary Data 7
DFT calculation for the optimized structures.
Supplementary Data 8
DFT calculation for the optimized structures.
Supplementary Data 9
DFT calculation for the optimized structures.
Supplementary Data 10
DFT calculation for the optimized structures.
Supplementary Data 11
DFT calculation for the optimized structures.
Supplementary Data 12
DFT calculation for the optimized structures.
Supplementary Data 13
DFT calculation for the optimized structures.
Source data
Source Data Fig. 1
DFT and XPS data used to plot Fig. 1e–h.
Source Data Fig. 2
Pearson coefficient data used to plot Fig. 2b,c,e,f.
Source Data Fig. 3
Electrochemical data used to plot Fig. 3a–d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raj, V., Wang, Y., Feng, M. et al. Grain boundary zirconia-modified garnet solid-state electrolyte. Nat. Mater. 25, 249–258 (2026). https://doi.org/10.1038/s41563-025-02374-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02374-9