Abstract
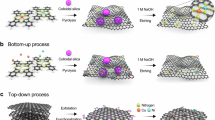
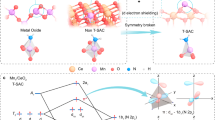
Dual-atom catalysts (DACs) exhibit high catalytic activity and metal utilization, alongside structural diversity with a wide range of catalytic site configurations. These features position DACs as promising candidates for energy conversion technologies. However, the precise control over atomic dispersion, pairing ratios and interatomic distances—which critically influence their multifunctional catalytic behavior—remains a formidable challenge. Here we developed a ligand-restricted strategy for the precise synthesis of highly paired DACs with tunable atomic distances. This was accomplished by coordinating diamine ligands with dual-metal precursors, restricting the pairing and relative positions of two metal atoms on two-dimensional graphitic carbon nitride. The atomic pairing ratio exceeded 82%, and the atomic pairing distance was controlled by the chain length of the diamine ligand. As a demonstration, the paired Pt1-Au1 DACs exhibited almost threefold enhancement in catalytic activity for nitrate reduction to ammonia compared with their unpaired counterparts. This work introduces an effective strategy for the atomic-scale fabrication of complex catalysts as well as provides valuable insights into nanoscale reaction mechanisms in heterogeneous catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data in this paper are presented in the article and Supplementary Information. Source data are provided with this paper.
References
Wang, A. et al. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Lang, R. et al. Single-atom catalysts based on the metal-oxide interaction. Chem. Rev. 120, 11986–12043 (2020).
Li, J. et al. Introduction: heterogeneous single-atom catalysis. Chem. Rev. 120, 11699–11702 (2020).
Zhang, S. et al. Atomically dispersed bimetallic Fe-Co electrocatalysts for green production of ammonia. Nat. Sustain. 6, 169–179 (2023).
Jung, E. et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Zhang, S. et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 14, 3634 (2023).
Li, R. & Wang, D. Superiority of dual-atom catalysts in electrocatalysis: one step further than single-atom catalysts. Adv. Energy Mater. 12, 2103564 (2022).
Huang, F. et al. Low-temperature acetylene semi-hydrogenation over the Pd1-Cu1 dual-atom catalyst. J. Am. Chem. Soc. 144, 18485–18493 (2022).
Ma, Y. F. et al. Single atom catalysts in liquid phase selective hydrogenations. Chem. Res. Chin. Univ. 38, 1163–1171 (2022).
Wei, X. et al. Cu acting as Fe activity promoter in dual-atom Cu/Fe-NC catalyst in CO2RR to C1 products. Appl. Surf. Sci. 564, 150423 (2021).
Liang, X.-M. et al. Controlled synthesis of a Ni2 dual-atom catalyst for synergistic CO2 electroreduction. Appl. Catal. B 322, 122073 (2023).
Jiao, J. et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem. 11, 222–228 (2019).
Li, R. & Wang, D. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res. 15, 6888–6923 (2022).
Liu, M. et al. Cascade synthesis of Fe-N2-Fe dual-atom catalysts for superior oxygen catalysis. Angew. Chem. Int. Ed. 63, e202408914 (2024).
Wei, Y. S. et al. Fabricating dual-atom iron catalysts for efficient oxygen evolution reaction: a heteroatom modulator approach. Angew. Chem. Int. Ed. 132, 2–11 (2020).
Lu, Z. et al. An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem. Int. Ed. 58, 2622–2626 (2019).
Ding, T. et al. Atomically precise dinuclear site active toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 11317–11324 (2021).
Zhang, Y. X. et al. General synthesis of a diatomic catalyst library via a macrocyclic precursor-mediated approach. J. Am. Chem. Soc. 145, 4819–4827 (2023).
Xie, P. et al. Oxo dicopper anchored on carbon nitride for selective oxidation of methane. Nat. Commun. 13, 1375 (2022).
Chen, C. et al. Adjacent Fe site boosts electrocatalytic oxygen evolution at Co site in single-atom-catalyst through a dual-metal-site design. Energy Environ. Sci. 16, 1685–1696 (2023).
Yu, D. et al. Dual-sites coordination engineering of single atom catalysts for flexible metal-air batteries. Adv. Energy Mater. 11, 2101242 (2021).
Zhang, L. et al. Enhanced oxygen reduction activity and stability of double-layer nitrogen-doped carbon catalyst with abundant Fe-Co dual-atom sites. Nano Energy 117, 108854 (2023).
Zhao, E. et al. Diatomic palladium catalyst for enhanced photocatalytic water-donating transfer hydrogenation. J. Am. Chem. Soc. 147, 2029–2036 (2025).
Wang, X. et al. Confined Fe-Cu clusters as sub-nanometer reactors for efficiently regulating the electrochemical nitrogen reduction reaction. Adv. Mater. 32, 2004382 (2020).
Gong, F. et al. Universal sub-nanoreactor strategy for synthesis of yolk-shell MoS2 supported single atom electrocatalysts toward robust hydrogen evolution reaction. Angew. Chem. Int. Ed. 62, e202308091 (2023).
Lu, J. Atomic Lego catalysts synthesized by atomic layer deposition. Acc. Mater. Res. 3, 358–368 (2022).
Yan, H. et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 8, 1070 (2017).
Ye, W. et al. Precisely tuning the number of Fe atoms in clusters on N-doped carbon toward acidic oxygen reduction reaction. Chem 5, 2865–2878 (2019).
Zhang, N. et al. A supported Pd2 dual-atom site catalyst for efficient electrochemical CO2 reduction. Angew. Chem. Int. Ed. 60, 13388–13393 (2021).
Li, Y. et al. Dual-atom Ag2/graphene catalyst for efficient electroreduction of CO2 to CO. Appl. Catal. B 268, 118747 (2020).
Tian, S. et al. Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation. Nat. Commun. 9, 2353 (2018).
Wang, J. et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017).
Wang, J. et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 11, 3375–3379 (2018).
Ren, W. et al. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew. Chem. Int. Ed. 58, 6972–6976 (2019).
Tang, T. et al. Dual-atom Co-Fe catalysts for oxygen reduction reaction. Chin. J. Catal. 46, 48–55 (2023).
Han, X. et al. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 31, 1905622 (2019).
Li, W. H. et al. Long-range interactions in diatomic catalysts boosting electrocatalysis. Angew. Chem. Int. Ed. 61, e202213318 (2022).
Meng, X. et al. Distance synergy of MoS2-confined rhodium atoms for highly efficient hydrogen evolution. Angew. Chem. Int. Ed. 132, 10588–10593 (2020).
Jiang, S. et al. Visualization of the distance-dependent synergistic interaction in heterogeneous dual-site catalysis. J. Am. Chem. Soc. 146, 29084–29093 (2024).
Ong, W. J. et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability?. Chem. Rev. 116, 7159–7329 (2016).
Tian, X. et al. Surface P atom grafting of g-C3N4 for improved local spatial charge separation and enhanced photocatalytic H2 production. J. Mater. Chem. A 7, 7628–7635 (2019).
Yang, X. et al. Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation. Appl. Catal. B 193, 22–35 (2016).
Wang, T. et al. Nature of metal-support interaction for metal catalysts on oxide supports. Science 386, 915–920 (2024).
Jiao, L. et al. Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction. J. Am. Chem. Soc. 143, 19417–19424 (2021).
Wang, B. et al. A general metal ion recognition strategy to mediate dual-atomic-site catalysts. J. Am. Chem. Soc. 146, 24945–24955 (2024).
Pan, X. & Bao, X. The effects of confinement inside carbon nanotubes on catalysis. Acc. Chem. Res. 44, 553–562 (2011).
Xiao, J. et al. Toward fundamentals of confined catalysis in carbon nanotubes. J. Am. Chem. Soc. 137, 477–482 (2015).
Su, J. et al. Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports. Nat. Catal. 6, 818–828 (2023).
Pan, X. et al. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 6, 507–511 (2007).
Liu, S. et al. Identify the activity origin of Pt single-atom catalyst via atom-by-atom counting. J. Am. Chem. Soc. 143, 15243–15249 (2021).
Ma, Y. F. et al. Tailoring of the proximity of platinum single atoms on CeO2 using phosphorus boosts the hydrogenation activity. ACS Catal. 9, 8404–8412 (2019).
Cao, S. et al. High-loading single Pt atom sites Pt-O(OH)x catalyze the CO PROX reaction with high activity and selectivity at mild conditions. Sci. Adv. 6, eaba3809 (2020).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Gao, R. et al. Pt/Fe2O3 with Pt-Fe pair sites as a catalyst for oxygen reduction with ultralow Pt loading. Nat. Energy 6, 614–623 (2021).
Zhang, B. et al. Stabilizing a platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity. Angew. Chem. Int. Ed. 55, 8319–8323 (2016).
Macino, M. et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene. Nat. Catal. 2, 873–881 (2019).
Sun, M. et al. Self-validated machine learning study of graphdiyne-based dual atomic catalyst. Adv. Energy Mater. 11, 2003796 (2021).
Chen, B. et al. Machine learning accelerated prediction of self-trapped excitons in double halide perovskites. Adv. Energy Sustain. Res. 4, 2370024 (2023).
Zecevic, J. et al. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 528, 245–248 (2015).
Acknowledgements
This research was financially supported by the National Key Research and Development Program of China (2022YFA1205200 to L.W.), the National Natural Science Foundation of China (52033003 to L.W., 22279139 to J.L., 62227815 to J.L., 22202027 to S.L. and 22572012 to S.L.), Program of Higher-Level Talents of IMU (10000-23112101/173 to J.L.), Project of Grassland Talent of Inner Mongolia Autonomous Region (12000-12102805 to J.L.) and the Natural Science Foundation of Inner Mongolia Autonomous Region of China (20241Q06 to J.L.). We thank C. Li, R. Che, J. Liang, P.-C. Chen, H. Tan, Y. Gu, W. Tu, J. Du and Y. Hao for helpful discussion.
Author information
Authors and Affiliations
Contributions
This study was conceived by J.L. and L.W. Y.M. carried out the synthesis, characterization and catalytic measurements. S.L. and W. Liu contributed to the electron-microscopy-based atom recognition statistics methodology. W.Z., R.Z., K.S. and R.G. performed the DFT calculations and analysis. W. Li carried out the catalytic performance. J.M. and Z.J. performed the XAS measurement and analysed the data. Y.M., Y.Z., S.L., J.S. and W. Liu performed the AC-HAADF-STEM characterization. M.S. and B.H. performed the DFT and ML. Y.M., G.Q.M.L., J.L. and L.W. wrote the paper. All authors were involved in discussions on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Huiyuan Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–46, Tables 1–6 and discussion.
Source data
Source Data Fig. 1
Source data for Fig. 1e,g,i–k.
Source Data Fig. 2
Source data for Fig. 2b–e,k–m.
Source Data Fig. 3
Source data for Fig. 3d–f.
Source Data Fig. 4
Source data for Fig. 4a–f.
Source Data Fig. 5
Source data for Fig. 5b–g.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Liu, S., Mao, J. et al. Ligand-restricted synthesis of highly paired dual-atom catalysts. Nat. Mater. 25, 80–90 (2026). https://doi.org/10.1038/s41563-025-02385-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02385-6