Abstract
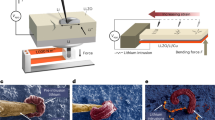
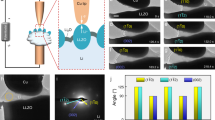
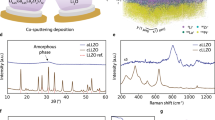
Lithium dendrite intrusion in solid-state batteries limits fast charging and causes short-circuiting, yet the underlying regulating mechanisms are not well-understood. Here we discover that heterogeneous Ag+ doping dramatically affects lithium intrusion into Li6.6La3Zr1.6Ta0.4O12 (LLZO), a brittle solid electrolyte. Nanoscale Ag+ doping is achieved by thermally annealing a 3-nm-thick metallic coating on LLZO, inducing Ag–Li ion exchange and Ag diffusion into grains and grain boundaries. Density functional theory calculations and experimental characterization show negligible impact on the electronic properties and surface wettability from Ag+ incorporation. Mechanically, nanoindentation experiments show a fivefold increase in the mechanical force required to fracture the surface Ag+-doped LLZO, indicating substantial doping-induced surface toughening. Operando microprobe scanning electron microscopy experiments show that the Ag+-doped LLZO surface exhibits improved lithium plating at >250 mA cm−2 and an electroplating diameter that is expanded by over fourfold, even under an extreme indentation stress of 3 GPa. This demonstrates enhanced defect tolerance in LLZO, rather than electronic or adhesion effects. Our study reveals a chemo-mechanical mechanism via surface heterogeneous doping, complementing present bulk design rules to minimize mechanical failures in solid-state batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data generated or analysed during this study are included in the Supplementary Information. Further data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Cao, D. et al. Lithium dendrite in all-solid-state batteries: growth mechanisms, suppression strategies, and characterizations. Matter 3, 57–94 (2020).
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Zhang, H., Ren, Y., Wu, X. & Wang, N. An interface-modified solid-state electrochemical device for lithium extraction from seawater. J. Power Sources 482, 228938 (2021).
Xiong, Y. et al. Electrochemical lithium extraction from aqueous sources. Matter 5, 1760–1791 (2022).
Teprovich, J. A. Jr et al. Electrochemical extraction of hydrogen isotopes from Li/LiT mixtures. Fusion Eng. Des. 139, 1–6 (2019).
Tsai, C.-L. et al. Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Appl. Mater. Interfaces 8, 10617–10626 (2016).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Ning, Z. et al. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature 618, 287–293 (2023).
McConohy, G. et al. Mechanical regulation of lithium intrusion probability in garnet solid electrolytes. Nat. Energy 8, 241–250 (2023).
Cheng, E. J., Sharafi, A. & Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 223, 85–91 (2017).
Shen, F., Dixit, M. B., Xiao, X. & Hatzell, K. B. Effect of pore connectivity on Li dendrite propagation within LLZO electrolytes observed with synchrotron X-ray tomography. ACS Energy Lett. 3, 1056–1061 (2018).
Tian, H.-K., Xu, B. & Qi, Y. Computational study of lithium nucleation tendency in Li7La3Zr2O12 (LLZO) and rational design of interlayer materials to prevent lithium dendrites. J. Power Sources 392, 79–86 (2018).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Tian, H.-K., Liu, Z., Ji, Y., Chen, L.-Q. & Qi, Y. Interfacial electronic properties dictate Li dendrite growth in solid electrolytes. Chem. Mater. 31, 7351–7359 (2019).
Liu, X. et al. Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021).
Xiao, Y. et al. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 5, 105–126 (2020).
Alexander, G. V., Shi, C., O’Neill, J. & Wachsman, E. D. Extreme lithium-metal cycling enabled by a mixed ion- and electron-conducting garnet three-dimensional architecture. Nat. Mater. 22, 1136–1143 (2023).
Dai, J., Yang, C., Wang, C., Pastel, G. & Hu, L. Interface engineering for garnet-based solid-state lithium-metal batteries: materials, structures, and characterization. Adv. Mater. 30, 1802068 (2018).
Feng, W., Dong, X., Li, P., Wang, Y. & Xia, Y. Interfacial modification of Li/garnet electrolyte by a lithiophilic and breathing interlayer. J. Power Sources 419, 91–98 (2019).
Kim, S. et al. The role of interlayer chemistry in Li-metal growth through a garnet-type solid electrolyte. Adv. Energy Mater. 10, 1903993 (2020).
Xiang, X. et al. Improving the interfacial contact between Li7La3Zr2O12 and lithium anode by depositing a film of silver. J. Electrochem. Soc. 168, 060515 (2021).
Qi, Y., Ban, C. & Harris, S. J. A new general paradigm for understanding and preventing Li metal penetration through solid electrolytes. Joule 4, 2599–2608 (2020).
Jagad, H. D., Harris, S. J., Sheldon, B. W. & Qi, Y. Ion size effects on the thermodynamic, kinetic, and mechanical properties during ion exchange in solid-state electrolytes. Chem. Mater. 37, 8165–8177 (2025).
Yao, X. et al. Xenon ion implantation induced surface compressive stress for preventing dendrite penetration in solid-state electrolytes. Small 18, 2108124 (2022).
Flatscher, F. et al. Deflecting dendrites by introducing compressive stress in Li7La3Zr2O12 using ion implantation. Small 20, 2307515 (2024).
Garfinkel, H. M. Ion-exchange equilibriums between glass and molten salts. J. Phys. Chem. 72, 4175–4181 (1968).
Shah, M. L. Optical waveguides in LiNbO3 by ion exchange technique. Appl. Phys. Lett. 26, 652–653 (1975).
Biesuz, M. et al. Solid-state field-assisted ion exchange of Ag in lithium aluminum silicate glass-ceramics: a superfast processing route toward stronger materials with antimicrobial properties. J. Eur. Ceram. Soc. 42, 1750–1761 (2022).
Ohtsuki, T., Peyghambarian, N., Honkanen, S. & Najafi, S. I. Gain characteristics of a high concentration Er3+-doped phosphate glass waveguide. J. Appl. Phys. 78, 3617–3621 (1995).
Krauskopf, T. et al. The fast charge transfer kinetics of the lithium metal anode on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. Adv. Energy Mater. 10, 2000945 (2020).
Zhao, J. et al. In situ observation of Li deposition-induced cracking in garnet solid electrolytes. Energy Environ. Mater. 5, 524–532 (2022).
Cui, C. et al. Unlocking the in situ Li plating dynamics and evolution mediated by diverse metallic substrates in all-solid-state batteries. Sci. Adv. 8, eadd2000 (2022).
Wei, Z. et al. In situ observation of room-temperature magnesium metal deposition on a NASICON/IL hybrid solid electrolyte. Adv. Energy Mater. 13, 2302525 (2023).
Siniscalchi, M. et al. Initiation of dendritic failure of LLZTO via sub-surface lithium deposition. Energy Environ. Sci. 17, 2431–2440 (2024).
Kondo, S., Mitsuma, T., Shibata, N. & Ikuhara, Y. Direct observation of individual dislocation interaction processes with grain boundaries. Sci. Adv. 2, e1501926 (2016).
Kondo, S., Ishihara, A., Tochigi, E., Shibata, N. & Ikuhara, Y. Direct observation of atomic-scale fracture path within ceramic grain boundary core. Nat. Commun. 10, 2112 (2019).
Alam, T. & Das, A. Review on impurity and conductivity issues of garnet type Li7La3Zr2O12: mechanisms, solutions, and perspectives. Energy Fuels 37, 15267–15282 (2023).
Jiang, X. et al. Effect of potassium and silver ion-exchange on the strengthening effect and properties of aluminosilicate glass. Ceram. Int. 49, 31351–31363 (2023).
Andersen, H. H. The depth resolution of sputter profiling. Appl. Phys. 18, 131–140 (1979).
Zalm, P. C. Ultra shallow doping profiling with SIMS. Rep. Prog. Phys. 58, 1321–1374 (1995).
Tan, J. & Tiwari, A. Fabrication and characterization of Li7La3Zr2O12 thin films for lithium ion battery. ECS Solid State Lett. 1, Q57–Q60 (2012).
Paolella, A. et al. Discovering the influence of lithium loss on garnet Li7La3Zr2O12 electrolyte phase stability. ACS Appl. Energy Mater. 3, 3415–3424 (2020).
Huang, X. et al. Developing preparation craft platform for solid electrolytes containing volatile components: experimental study of competition between lithium loss and densification in Li7La3Zr2O12. ACS Appl. Mater. Interfaces 14, 33340–33354 (2022).
Hale, D. K. Strengthening of silicate glasses by ion exchange. Nature 217, 1115–1118 (1968).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Callister, W. D. Jr & Rethwisch, D. G. Materials Science and Engineering (Wiley, 2019).
Yu, S. & Siegel, D. J. Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 29, 9639–9647 (2017).
Gao, B., Jalem, R., Tian, H.-K. & Tateyama, Y. Revealing atomic-scale ionic stability and transport around grain boundaries of garnet Li7La3Zr2O12 solid electrolyte. Adv. Energy Mater. 12, 2102151 (2022).
Cojocaru-Mirédin, O. et al. Quantifying lithium enrichment at grain boundaries in Li7La3Zr2O12 solid electrolyte by correlative microscopy. J. Power Sources 539, 231417 (2022).
Zhu, Y. et al. Dopant-dependent stability of garnet solid electrolyte interfaces with lithium metal. Adv. Energy Mater. 9, 1803440 (2019).
Yu, S. et al. Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 28, 197–206 (2016).
Xie, H., Alonso, J. A., Li, Y., Fernández-Díaz, M. T. & Goodenough, J. B. Lithium distribution in aluminum-free cubic Li7La3Zr2O12. Chem. Mater. 23, 3587–3589 (2011).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Das, T., Nicholas, J. D. & Qi, Y. Long-range charge transfer and oxygen vacancy interactions in strontium ferrite. J. Mater. Chem. A 5, 4493–4506 (2017).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency, Vehicle Technologies Office of the US Department of Energy under the Advanced Battery Materials Research Program. Additional support was provided by Samsung Advanced Institute of Technology. T.C., S.S.L. and X.W.G. acknowledge financial support from the StorageX Initiative at Stanford University and the grant DE-SC0021075 funded by the US Department of Energy, Office of Science. X.X. acknowledge additional financial support from the Ira A. Fulton Schools of Engineering at Arizona State University and the grant DE-SC0026366 funded by the US Department of Energy, Office of Science. Y.C. (0000-0002-6103-6352) acknowledges cryo-EM support from the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under contract DE-AC02-76SF00515. H.D.J. and Y.Q. acknowledge financial support from the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy through the Battery 500 Consortium. We thank W. D. Nix for his contributions, including discussions and the examination of nanoindentation data and mechanical analyses; Y. Ju for discussion on the Li-plating dynamics on solid electrolytes; R. Chin, J. Jamtgaard, P. Wallace, C. Jilly-Rehak and M. Mills for assistance with the Helios SEM/FIB and nanoSIMS instruments; and L. Miara, S. Chakravarthy, S. J. Harris, A. Lee, Y. Liu and J. A. Greer for discussions. We extend special thanks to C. Zhao for his contribution to the art design of the schematics used in this work. Finally, we thank J.-H. Lee for discussions and comments on the manuscript. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation under award ECCS-2026822.
Author information
Authors and Affiliations
Contributions
X.X. performed all of the experiments and analysed the results, with contributions from T.C., G.M. and S.S.L. Support in the XPS analysis was provided by S.W. and Y.Y. Contributions to the TEM characterization and analysis were made by Y.C. (0000-0001-8219-1856), Z.Z., H.R.L., R.S. and Y.C. (0000-0002-6103-6352). S.S.L., M.M.W. and R.X. contributed to the nanoindentation measurements. E.B. contributed to preparation of the LLZO samples. E.K., L.N., A.R. and A.G. contributed to electrical measurements and data analysis. C.M. and L.H. contributed to the surface coatings. H.D.J. and Y.Q. performed the DFT calculations on the Ag–Li ion-exchange process. X.W.G. supervised the nanoindentation measurements and their analysis. X.X. and W.C.C. designed the research plan and supervised the work. X.X. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–45.
Source data
Source Data Fig. 1
Depth-profiling XPS data for Ag, Zr and La plotted in Fig. 1c; depth profiling nanoSIMS data for Ag0 | LLZO and Ag+-LLZO plotted in Fig. 1d.
Source Data Fig. 2
DFT density of states data for Ag, Li, La, Oneighbour, Onon-neighbour and Zr plotted in Fig. 2b–g.
Source Data Fig. 4
Operando SEM electrochemical data of the lateral diameter of Li electroplating and the local current density at failure in various LLZO samples plotted in Fig. 4b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Cui, T., McConohy, G. et al. Heterogeneous doping via nanoscale coating impacts the mechanics of Li intrusion in brittle solid electrolytes. Nat. Mater. (2026). https://doi.org/10.1038/s41563-025-02465-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41563-025-02465-7