Abstract
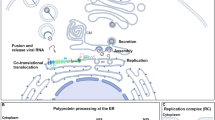
The endoplasmic reticulum (ER) is an architecturally diverse organelle that serves as a membrane source for the replication of multiple viruses. Flaviviruses, including yellow fever virus, West Nile virus, dengue virus and Zika virus, induce unique single-membrane ER invaginations that house the viral replication machinery1. Whether this virus-induced ER remodelling is vulnerable to antiviral pathways is unknown. Here, we show that flavivirus replication at the ER is targeted by the interferon (IFN) response. Through genome-scale CRISPR screening, we uncovered an antiviral mechanism mediated by a functional gene pairing between IFI6 (encoding IFN-α-inducible protein 6), an IFN-stimulated gene cloned over 30 years ago2, and HSPA5, which encodes the ER-resident heat shock protein 70 chaperone BiP. We reveal that IFI6 is an ER-localized integral membrane effector that is stabilized through interactions with BiP. Mechanistically, IFI6 prophylactically protects uninfected cells by preventing the formation of virus-induced ER membrane invaginations. Notably, IFI6 has little effect on other mammalian RNA viruses, including the related Flaviviridae family member hepatitis C virus, which replicates in double-membrane vesicles that protrude outwards from the ER. These findings support a model in which the IFN response is armed with a membrane-targeted effector that discriminately blocks the establishment of virus-specific ER microenvironments that are required for replication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data sets generated and/or analysed during the current study are available from the corresponding author upon request.
References
Neufeldt, C. J., Cortese, M., Acosta, E. G. & Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 16, 125–142 (2018).
Kelly, J. M. et al. Characterization of a human gene inducible by alpha- and beta-interferons and its expression in mouse cells. EMBO J. 5, 1601–1606 (1986).
Shresta, S. et al. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 78, 2701–2710 (2004).
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Schoggins, J. W. et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695 (2014).
Li, J. et al. A short hairpin RNA screen of interferon-stimulated genes identifies a novel negative regulator of the cellular antiviral response. mBio 4, e00385-13 (2013).
Schoggins, J. W. et al. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl Acad. Sci. USA 109, 14610–14615 (2012).
Parker, N. & Porter, A. C. Identification of a novel gene family that includes the interferon-inducible human genes 6-16 and ISG12. BMC Genomics 5, 8 (2004).
Gjermandsen, I. M., Justesen, J. & Martensen, P. M. The interferon-induced gene ISG12 is regulated by various cytokines as the gene 6-16 in human cell lines. Cytokine 12, 233–238 (2000).
Wang, J., Lee, J., Liem, D. & Ping, P. HSPA5 gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 618, 14–23 (2017).
Gaut, J. R. & Hendershot, L. M. Mutations within the nucleotide binding site of immunoglobulin-binding protein inhibit ATPase activity and interfere with release of immunoglobulin heavy chain. J. Biol. Chem. 268, 7248–7255 (1993).
Munro, S. & Pelham, H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46, 291–300 (1986).
Lackner, D. H. et al. A generic strategy for CRISPR–Cas9-mediated gene tagging. Nat. Commun. 6, 10237 (2015).
Cheriyath, V. et al. G1P3, an IFN-induced survival factor, antagonizes TRAIL-induced apoptosis in human myeloma cells. J. Clin. Invest. 117, 3107–3117 (2007).
Cheriyath, V. et al. G1P3, an interferon- and estrogen-induced survival protein contributes to hyperplasia, tamoxifen resistance and poor outcomes in breast cancer. Oncogene 31, 2222–2236 (2012).
Tahara, E. et al. G1P3, an interferon inducible gene 6-16, is expressed in gastric cancers and inhibits mitochondrial-mediated apoptosis in gastric cancer cell line TMK-1 cell. Cancer Immunol. Immunother. 54, 729–740 (2005).
Miller, S., Sparacio, S. & Bartenschlager, R. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J. Biol. Chem. 281, 8854–8863 (2006).
Vogt, D. A. & Ott, M. Membrane flotation assay. Bio. Protoc. 5, e1435 (2015).
Fujiki, Y., Hubbard, A. L., Fowler, S. & Lazarow, P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 93, 97–102 (1982).
Jones, C. T., Patkar, C. G. & Kuhn, R. J. Construction and applications of yellow fever virus replicons. Virology 331, 247–259 (2005).
Aguirre, S. et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 8, e1002934 (2012).
Zhang, R. et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 (2016).
Gillespie, L. K., Hoenen, A., Morgan, G. & Mackenzie, J. M. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J. Virol. 84, 10438–10447 (2010).
Roosendaal, J., Westaway, E. G., Khromykh, A. & Mackenzie, J. M. Regulated cleavages at the West Nile virus NS4A-2K-NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein. J. Virol. 80, 4623–4632 (2006).
Miller, S., Kastner, S., Krijnse-Locker, J., Buhler, S. & Bartenschlager, R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 282, 8873–8882 (2007).
Qi, H. et al. Systematic identification of anti-interferon function on hepatitis C virus genome reveals p7 as an immune evasion protein. Proc. Natl Acad. Sci. USA 114, 2018–2023 (2017).
Itsui, Y. et al. Expressional screening of interferon-stimulated genes for antiviral activity against hepatitis C virus replication. J. Viral Hepat. 13, 690–700 (2006).
Meyer, K. et al. Interferon-α inducible protein 6 impairs EGFR activation by CD81 and inhibits hepatitis C virus infection. Sci. Rep. 5, 9012 (2015).
Metz, P. et al. Identification of type I and type II interferon-induced effectors controlling hepatitis C virus replication. Hepatology 56, 2082–2093 (2012).
Fusco, D. N. et al. A genetic screen identifies interferon-α effector genes required to suppress hepatitis C virus replication. Gastroenterology 144, 1438–1449 (2013).
Zhao, H. et al. A functional genomic screen reveals novel host genes that mediate interferon-alpha’s effects against hepatitis C virus. J. Hepatol. 56, 326–333 (2012).
Knoops, K. et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 6, e226 (2008).
Hanners, N. W. et al. Western Zika virus in human fetal neural progenitors persists long term with partial cytopathic and limited immunogenic effects. Cell Rep. 15, 2315–2322 (2016).
Marukian, S. et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology 48, 1843–1850 (2008).
Schwarz, M. C. et al. Rescue of the 1947 Zika virus prototype strain with a Cytomegalovirus promoter-driven cDNA clone. mSphere 1, e00246-16 (2016).
Grigorov, B., Rabilloud, J., Lawrence, P. & Gerlier, D. Rapid titration of measles and other viruses: optimization with determination of replication cycle length. PLoS ONE 6, e24135 (2011).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Perelman, S. S. et al. Cell-based screen identifies human interferon-stimulated regulators of Listeria monocytogenes infection. PLoS Pathog. 12, e1006102 (2016).
Acknowledgements
We thank N. Alto and J. Pfeiffer for critical manuscript feedback, M. Diamond and M. Henne for helpful discussions and C. Rice for reagents. We acknowledge the technical support of the UT Southwestern Live Cell Imaging Core and Electron Microscopy Core. This work was supported in part by NIH grants AI117922 and DK095031 (J.W.S.), the UT Southwestern Endowed Scholars program (J.W.S.), the Rita Allen Foundation (J.W.S.), the Clayton Foundation for Research (J.W.S.) and the National Science Foundation Graduate Research Fellowship Program grant 2016217834 (I.N.B.). C.X. was partially supported by NIH grant UL1TR001105. Additional support for R.B.R. and K.B.M. was obtained from NIH T32 Training grants AI007520 and AI005284, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the other funders.
Author information
Authors and Affiliations
Contributions
R.B.R., M.B.O. and J.W.S. designed the project. R.B.R., M.B.O., J.L.E., M.B.M., I.N.B., K.B.M., P.C.D.-R. and J.W.S. performed the experimental work. C.D. and G.K. provided the human fetal neural progenitor cultures. All authors contributed to data analysis and interpretation. A.K. and C.X. processed the CRISPR data sets. R.B.R., M.B.O. and J.W.S. drafted the manuscript text. All authors contributed to the editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–9, Supplementary Tables 1 and 2, Supplementary Methods and Supplementary References.
Supplementary Table 1
MAGeCK analysis output of CRISPR screen.
Rights and permissions
About this article
Cite this article
Richardson, R.B., Ohlson, M.B., Eitson, J.L. et al. A CRISPR screen identifies IFI6 as an ER-resident interferon effector that blocks flavivirus replication. Nat Microbiol 3, 1214–1223 (2018). https://doi.org/10.1038/s41564-018-0244-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-018-0244-1
This article is cited by
-
The first trimester human placenta responds to Zika virus infection inducing an interferon (IFN) and antiviral interferon stimulated gene (ISG) response
Virology Journal (2025)
-
Zika virus inhibits mitophagy to facilitate its replication
Animal Diseases (2025)
-
Recapitulating dengue virus infection with human pluripotent stem cell-derived liver organoids for antiviral screening
Nature Communications (2025)
-
Role of innate immune and inflammatory signaling in West Nile virus tropism and neuronal and glial cell death
Scientific Reports (2025)
-
Systematically Investigating CRISPR/Cas12a Fluorescent Biosensor for Sensitive and Specific Single Nucleotide Variants Detection
Journal of Fluorescence (2025)