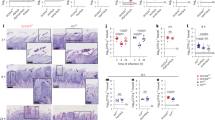
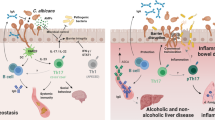
Abstract
Candida albicans is a common resident of the microbiota that supports host homeostasis but can cause disease when immune defences are impaired. Mucocutaneous candidiasis in individuals with IL-17 immune defects provides insights into the immune system’s role in controlling C. albicans. Here, using a murine model of oral colonization, we show that IL-17 signalling maintains C. albicans in a non-pathogenic state. Loss of IL-17 leads to fungal filamentation and upregulation of hyphae-associated genes, which is accompanied by epithelial barrier disruption and inflammation, linked to aberrant IL-22 and IL-13 production. The emergence of pathogenic fungal traits was associated with impaired zinc chelation due to reduced calprotectin expression in the IL-17-deficient mice. Prolonged exposure to the immune-dysregulated tissue led to selection of stable, damage-inducing C. albicans variants, mirroring the evolution of isolates from a chronic mucocutaneous candidiasis patient. These findings reveal how IL-17 protects against fungal pathogenicity and how immune dysfunction fosters C. albicans adaptation and diversification within the host.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are publicly available via Zenodo at https://doi.org/10.5281/zenodo.17233074 ref. 90. RNA-seq datasets generated in this study are available at NCBI (GEO repository GSE280210).
References
Leonardi, I. et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 185, 831–846.e14 (2022).
Shao, T. Y. et al. Commensal Candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe 25, 404–417.e6 (2019).
Tso, G. H. W. et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595 (2018).
Li, X. V., Leonardi, I. & Iliev, I. D. Gut mycobiota in immunity and inflammatory disease. Immunity 50, 1365–1379 (2019).
Zeng, S. et al. Candida albicans-specific Th17 cell-mediated response contributes to alcohol-associated liver disease. Cell Host Microbe 31, 389–404.e7 (2023).
Bacher, P. et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell 176, 1340–1355.e15 (2019).
Lu, S. Y. Oral candidosis: pathophysiology and best practice for diagnosis, classification, and successful management. J. Fungi 7, 555 (2021).
Denning, D. W., Kneale, M., Sobel, J. D. & Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis. 18, e339–e347 (2018).
Denning, D. W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(23)00692-8 (2024).
Fróis-Martins, R., Lagler, J. & LeibundGut-Landmann, S. Candida albicans virulence traits in commensalism and disease. Curr. Clin. Microbiol. Rep. https://doi.org/10.1007/s40588-024-00235-8 (2024).
d’Enfert, C. et al. The impact of the fungus–host–microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol. Rev. 45, fuaa060 (2021).
Kirchner, F. R. & LeibundGut-Landmann, S. Tissue-resident memory Th17 cells maintain stable fungal commensalism in the oral mucosa. Mucosal Immunol. 14, 455–467 (2021).
Park, C. O. et al. Staged development of long-lived T-cell receptor alphabeta T(H)17 resident memory T-cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol. 142, 647–662 (2018).
Kirchner, F. R. et al. Persistence of Candida albicans in the oral mucosa induces a curbed inflammatory host response that is independent of immunosuppression. Front. Immunol. 10, 330 (2019).
Schonherr, F. A. et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol. 10, 1335–1350 (2017).
Lionakis, M. S., Drummond, R. A. & Hohl, T. M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 23, 433–452 (2023).
Conti, H. R. et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311 (2009).
Anderson, F. M. et al. Candida albicans selection for human commensalism results in substantial within-host diversity without decreasing fitness for invasive disease. PLoS Biol. 21, e3001822 (2023).
Puel, A. et al. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr. Opin. Immunol. 22, 467–474 (2010).
Bougnoux, M. E. et al. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med. 34, 292–299 (2008).
Zelante, T. et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat. Commun. 3, 683 (2012).
Glocker, E. O. et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735 (2009).
Luo, G. et al. A human commensal-pathogenic fungus suppresses host immunity via targeting TBK1. Cell Host Microbe 32, 1536–1551.e6 (2024).
Majer, O. et al. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 8, e1002811 (2012).
Pekmezovic, M. et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat. Microbiol. 6, 643–657 (2021).
Li, T., Niu, X., Zhang, X., Wang, S. & Liu, Z. Recombinant human IFNα-2b response promotes vaginal epithelial cells defense against Candida albicans. Front. Microbiol. 8, 697 (2017).
Break, T. J. et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, eaay5731 (2021).
Piehler, D. et al. The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal Immunol. 9, 937–949 (2016).
Aggor, F. E. Y. et al. Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci. Immunol. 5, eaba0570 (2020).
Millet, N. et al. Non-canonical IL-22 receptor signaling remodels the mucosal barrier during fungal immunosurveillance. Preprint at bioRxiv https://doi.org/10.1101/2024.09.08.611873 (2024).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Chiricozzi, A. et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131, 677–687 (2011).
Conti, H. R. et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 20, 606–617 (2016).
Krishnakumari, V., Rangaraj, N. & Nagaraj, R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob. Agents Chemother. 53, 256–260 (2009).
Jukic, A., Bakiri, L., Wagner, E. F., Tilg, H. & Adolph, T. E. Calprotectin: from biomarker to biological function. Gut 70, 1978–1988 (2021).
Christmann, C. et al. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front. Immunol. 11, 599947 (2020).
Trautwein-Weidner, K., Gladiator, A., Nur, S., Diethelm, P. & LeibundGut-Landmann, S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 8, 221–231 (2015).
Zygiel, E. M. & Nolan, E. M. Transition metal sequestration by the host-defense protein calprotectin. Annu. Rev. Biochem. 87, 621–643 (2018).
Citiulo, F. et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 8, e1002777 (2012).
Loboda, D. & Rowinska-Zyrek, M. Zinc binding sites in Pra1, a zincophore from Candida albicans. Dalton Trans. 46, 13695–13703 (2017).
Alamir, O. F., Oladele, R. O. & Ibe, C. Nutritional immunity: targeting fungal zinc homeostasis. Heliyon 7, e07805 (2021).
Roselletti, E. et al. Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci. Transl. Med. 15, eadi3363 (2023).
Besold, A. N. et al. Role of calprotectin in withholding zinc and copper from Candida albicans. Infect. Immun. 86, e00779-17 (2018).
Clark, H. L. et al. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J. Immunol. 196, 336–344 (2016).
Cho, Y. E. et al. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 1, 29–35 (2007).
Sitterlé, E. La candidose cutanéo-muqueuse chronique: un modèle d’étude de l’adaptation génomique chez Candida albicans. Doctoral thesis, Doctoral school BioScience Paris Cité (2018).
Puel, A. et al. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr. Opin. Allergy Clin. Immunol. 12, 616–622 (2012).
Murdoch, C. C. & Skaar, E. P. Nutritional immunity: the battle for nutrient metals at the host–pathogen interface. Nat. Rev. Microbiol. 20, 657–670 (2022).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012).
Naik, S. et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Solis, N. V., Wakade, R. S., Filler, S. G. & Krysan, D. J. Candida albicans oropharyngeal infection is an exception to iron-based nutritional immunity. mBio 14, e0009523 (2023).
Wolk, K. et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36, 1309–1323 (2006).
Nograles, K. E. et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 159, 1092–1102 (2008).
Dalessandri, T., Crawford, G., Hayes, M., Castro Seoane, R. & Strid, J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat. Commun. 7, 12080 (2016).
Dixon, B. R., Radin, J. N., Piazuelo, M. B., Contreras, D. C. & Algood, H. M. IL-17a and IL-22 induce expression of antimicrobials in gastrointestinal epithelial cells and may contribute to epithelial cell defense against Helicobacter pylori. PLoS ONE 11, e0148514 (2016).
Pettas, E., Savva, V., Theofilou, V. I., Georgaki, M. & Nikitakis, N. G. Oral Candida infection in psoriatic patients treated with IL17A inhibitors: report of 3 cases and a comprehensive review of the literature. Diagnostics 12, 3 (2021).
Huang, M. Y., Woolford, C. A., May, G., McManus, C. J. & Mitchell, A. P. Circuit diversification in a biofilm regulatory network. PLoS Pathog. 15, e1007787 (2019).
Bartell, J. A. et al. Evolutionary highways to persistent bacterial infection. Nat. Commun. 10, 629 (2019).
Wartenberg, A. et al. Microevolution of Candida albicans in macrophages restores filamentation in a nonfilamentous mutant. PLoS Genet. 10, e1004824 (2014).
Pradhan, A. et al. Anticipatory stress responses and immune evasion in fungal pathogens. Trends Microbiol. 29, 416–427 (2021).
Ene, I. V. et al. Global analysis of mutations driving microevolution of a heterozygous diploid fungal pathogen. Proc. Natl Acad. Sci. USA 115, E8688–E8697 (2018).
Zenewicz, L. A. IL-22 binding protein (IL-22BP) in the regulation of IL-22 biology. Front. Immunol. 12, 766586 (2021).
Donnelly, R. P. et al. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 21, 393–401 (2010).
Ropars, J. et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans. Nat. Commun. 9, 2253 (2018).
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455 (2019).
Letunic, I. & Bork, P. Interactive Tree of Life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 52, W78–W82 (2024).
Rupniak, H. T. et al. Characteristics of four new human cell lines derived from squamous cell carcinomas of the head and neck. J. Natl Cancer Inst. 75, 621–635 (1985).
Parikh, N., Nagarajan, P., Sei-ichi, M., Sinha, S. & Garrett-Sinha, L. A. Isolation and characterization of an immortalized oral keratinocyte cell line of mouse origin. Arch. Oral. Biol. 53, 1091–1100 (2008).
Wachtler, B., Wilson, D., Haedicke, K., Dalle, F. & Hube, B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS ONE 6, e17046 (2011).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Noben-Trauth, N. et al. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl Acad. Sci. USA 94, 10838–10843 (1997).
Muller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Huang, S. et al. Immune response in mice that lack the interferon-gamma receptor. Science 259, 1742–1745 (1993).
Haas, J. D. et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37, 48–59 (2012).
Kreymborg, K. et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104 (2007).
Sparber, F. et al. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25, 389–403.e6 (2019).
Blache, D., Martin, G. B. & Maloney, S. K. Towards ethically improved animal experimentation in the study of animal reproduction. Reprod. Domest. Anim. 43, 8–14 (2008).
Solis, N. V. & Filler, S. G. Mouse model of oropharyngeal candidiasis. Nat. Protoc. 7, 637–642 (2012).
Cossarizza, A. et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 49, 1457–1973 (2019).
Hatakeyama, M. et al. SUSHI: an exquisite recipe for fully documented, reproducible and reusable NGS data analysis. BMC Bioinformatics 17, 228 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Frankish, A. et al. Gencode 2021. Nucleic Acids Res. 49, D916–D923 (2021).
Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Frois-Martins, R. et al. Genome-guided manipulation of regulators of morphogenesis in a C. albicans strain with low virulence is not sufficient to trigger a high-virulence phenotype. Preprint at bioRxiv https://doi.org/10.1101/2025.07.16.665085 (2025).
Lemberg, C. et al. Candida albicans commensalism in the oral mucosa is favoured by limited virulence and metabolic adaptation. PLoS Pathog. 18, e1010012 (2022).
Fróis Martins, R. & LeibundGut-Landmann, S. Raw dataset associated with the publication ‘IL-17-mediated antifungal immunity restricts Candida albicans pathogenicity in the oral cavity’. Zenodo https://doi.org/10.5281/zenodo.17233074 (2025).
Gillum, A. M., Tsay, E. Y. & Kirsch, D. R. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198, 179–182 (1984).
Acknowledgements
We thank the staff of the Laboratory Animal Service Center of the University of Zürich for animal husbandry; staff of the Laboratory for Animal Model Pathology of the University of Zürich for histology; the Functional Genomic Center of the University of Zürich for RNA-seq data acquisition and analysis; the Center for Clinical Studies (ZKS) of the University of Zürich for access to equipment; and members of the LeibundGut lab for helpful advice and discussions. This work was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie action, Innovative Training Network: FunHoMic (grant no. 812969 to S.L.-L. and C.d’E.), the Novartis Foundation for Medical-Biological Research (grant no. 22C224 to S.L.-L.), and a UZH Candoc grant (to R.F.-M.). Work in the laboratory of C.d’E. was supported by the Agence Nationale de Recherche (ANR-10-LABX-62-IBEID).
Author information
Authors and Affiliations
Contributions
R.F.-M. and S.L.-L. designed the study and wrote the paper. R.F.-M. performed the experiments and analysed the data. K.M.d.S.V. contributed to the experiments shown in Extended Data Fig. 4. S.M. conducted the experiment shown in Fig. 4a. C.M. and C.d’E. conducted the bioinformatic analyses. N.S., E.S., M.-E.B. and C.d’E. provided the C. albicans clinical isolates and related experimental data. S.M., N.S. and E.S. made equal contributions to the experimental work. S.L.-L. oversaw the study design and data analysis. R.F.-M., C.d’E. and S.L.-L. acquired the funding. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dysregulated immune and tissue homeostasis in the tongue of C. albicans-colonized Il17rc−/− mice.
IL17rc−/− and heterozygous littermate control mice were associated with C. albicans strain 101 via sublingual administration. A. Body weight kinetics (n = 4 / group, mean ± SD). B. Heat map of differentially expressed cytokine genes on day 3 and 19 (n = 3 / group). C.–E. Quantification of the indicated transcripts in the colonized tongue on day 19 (n = 7 or 9 / group, mean ± SEM, data pooled from 3 independent experiments, except for Cxcl2 and Krt14 where n = 4, 5 or 6 / group, mean ± SEM, data pooled from 2 independent experiments. The grey shaded area represents the expression levels of each gene in naïve animals. The statistical significance of differences between groups was determined by Two-way ANOVA (A) while in (C–E) two-sided unpaired t-test was use in all genes analyzed except Krt10 which statistical significance was determined by Mann-Whitney test.
Extended Data Fig. 2 Flow cytometry analysis of immune cell populations in the C. albicans-colonized tongue and draining lymph nodes.
A. – B. Gating strategy for neutrophils, monocytes (A), and cytokine-producing T cells (B) in the C. albicans-colonized tongue on day 19. C. – D. Gating strategy (C) and quantification (D) of cytokine-producing T cells in the cervical lymph nodes on day 19. In D, each symbol represents an individual mouse; the mean ± SEM per group is indicated; n = 6 or 8/ group, pooled from 2 independent experiments. The statistical significance of differences between groups was determined by two-sided unpaired t-test (single-positive populations) or two-sided Mann-Whitney test (double-positive population).
Extended Data Fig. 3 Tissue pathology is sustained by the presence of C. albicans.
A. – B. IL17rc−/− and heterozygous littermate control mice were associated with C. albicans strain 101 for 75 days. PAS-stained tongue sections (A; representative of 4 mice / group from one independent experiment; Scale bars: 250 μm; 25 μm or 50 μm for the inserts) and quantification of the indicated transcripts in the colonized tongue (B, n = 4 / group, mean ± SD). The grey shaded area represents the expression levels of each gene in naïve animals. C. – E. IL17rc−/− and heterozygous littermate control mice associated with C. albicans strain 101 were treated with fluconazole from day 14 to the endpoint on day 19. Tongue CFUs (C), PAS-stained tongue sections (D; representative of 4 and 9 mice from 2 independent experiments; Scale bars: 250 μm; 25 μm or 50 μm for the inserts), and quantification of the indicated transcripts in the colonized tongue (E, n = 4 or 9 / group, mean ± SEM, data pooled from 2 independent experiments). F. – G. IL17rc−/− and heterozygous littermate control mice associated with C. albicans strain 101WT (50x reduced infection dose, l.d.) or 101ece1Δ/Δ for 19 days. Transcript levels of Krt10, Dsgl4 and Slurp2 in the colonized tongue (F, n = 4 or 5, mean ± SD) and PAS-stained tongue sections (G; representative of 10 and 12 mice / group from 3 independent experiments; Scale bars: 100 μm; 50 μm for the inserts). The grey shaded area represents the expression levels of each gene in naïve animals. The statistical significance of differences between groups was determined by two-sided unpaired t-test (B, E), or two-sided Mann-Whitney test (C). ns, not significant (p ≥ 0.05).
Extended Data Fig. 4 Characterization of the commensal C. albicans isolates CEC3672 and CEC3678.
A. Colony morphology on Spider agar. B. Quantification of the hyphal length after exposure of the indicated strains to TR146 oral keratinocytes for 3.5 h (n = 200 filaments / group for strains CEC3672 and CEC3678; n = 90 filaments / group for strains SC5314 and 101, mean ± SD). C. LDH release from TR146 oral keratinocytes after exposure to the indicated strains for 24 h (n = 16 / group, mean ± SEM, data pooled from two independent experiments). D. Quantification of IL-1α in the supernatant of TR146 oral keratinocytes after exposure to the indicated strains for 24 h (n = 6 / group, mean ± SD). E. CFUs in the tongue of wildtype mice that were colonized with CEC3672 or CEC3678 for 1, 6 or 30 days. Each symbol is the mean ± SEM per group (n = 3, 5 or 6 / group pooled from 2 independent experiments). F. PAS-stained tongue sections of wild-type mice that were colonized with CEC3672 or CEC3678 for 1 day (representative of 6 mice / group from 2 independent experiments). Scale bars: 100 μm. In B, C, and D, the statistical significance of differences between groups was determined by One-way ANOVA. ns, not significant (p ≥ 0.05).
Extended Data Fig. 5 The acquired fungal virulence is not explained by aberrant IFN-I, IFN-γ or IL-13 expression.
Il17rc−/− Ifnar−/−, Il17rc−/− Ifngr−/− or Il17rc−/− Il4ra−/− double knockout mice and the respective heterozygous littermate control mice (Il17rc−/−) were associated with C. albicans strain 101 for 19 days. A. Tongue CFUs (n = 7, 8 or 9 / group, mean ± SEM, data pooled from 2-3 independent experiments). B. PAS-stained tongue sections (representative of at least 7 mice / group from 2-3 independent experiments). Scale bars: 100 μm; 25 μm for the inserts. C.–H. Il22, Krt10, and Dsg4 host transcripts and ECE1, HWP1, and SAP5 fungal transcripts in the colonized tongue as indicated. n = 4, 5, 7, 8 or 9 / group, mean ± SEM, data pooled from 2-3 independent experiments (or, for some genes n = 3 from one representative experiment, mean ± SD). The grey shaded area represents the expression levels of each host gene in naïve animals. The statistical significance of differences between groups was determined by two-sided Mann-Whitney (A, left panel) or two-sided unpaired t-test (A, middle and right panels, and C–H). ns, not significant (p ≥ 0.05).
Extended Data Fig. 6 IL-17 restricts C. albicans pathogenicity via the regulation of calprotectin.
A. IL17rc−/− mice were associated with C. albicans strain 101 via sublingual administration and ECE1, HWP1, SAP5 fungal transcripts were evaluated on day 3, 7 and 19 (n = 3 or 4 / group, mean ± SD). B.–C. IL17rc−/− and heterozygous littermate control mice were associated with C. albicans strain 101. B. Krt10, Dsg4, Slurp1 and Dsg1 expression in the colonized tongue on day 7 and 14 (n = 4 or 5 / group, mean ± SEM, data pooled from 2 independent experiments). The grey shaded area represents the expression levels of each gene in naïve animals. C. PAS-stained tongue tissue sections on day 7 (representative of 4 mice / group from 2 independent experiments; scale bars: 50 μm). D.–E. IL17rc−/− and heterozygous littermate control mice naïve mice PAS-stained tongue sections (D; representative of 5 mice / group from 2 independent experiments), expression of epithelial structural genes (E, n = 5 / group, mean ± SEM, data pooled from 2 independent experiments). F. Heat map of differentially expressed AMP genes in the tongue of IL17rc−/− and heterozygous littermate control mice on day 3 and day 19 (n = 3 / group). G. PRA1 expression by strain 101 upon exposure to recombinant calprotectin for 3.5-4 h (n = 4 / group, mean ± SEM, data pooled from 2 independent experiments). H. Representative images of C. albicans strains CEC3672 and CEC3678 in F12 medium for 3.5 - 4 h. I.–J. Filamentation of C. albicans strains CEC3672 and CEC3678 when put in contact with IMOK cells and supplemented with TPEN and ZnSO4, as indicated. Representative images (I) and quantification of hyphae length and IMOK cell invasion (J) after 3.5 - 4 h of exposure. Each symbol represents a hyphae filament (J, left panel; quantification of > 60 fungal cells / group, mean ± SD) or the percentage of hyphae invasion per analyzed image (J, right panel; n = 5 / group, mean ± SD). The statistical significance of differences between groups was determined by Two-way ANOVA (B), two-sided Mann-Whitney test (G), Kruskal-Wallis test (F left panel) or One-way ANOVA (A and F right panel). ns, not significant (p ≥ 0.05).
Extended Data Fig. 7 Phylogenetic tree for C. albicans strains used in this study.
Tree representing the phylogenetic relationships between 182 isolates representative of the C. albicans population, strain 101 and two mouse-evolved isolates derived from strain 101, namely Evo1 and Evo2 (shown in green) and strains CEC4511, CEC4512, CEC4513 and CEC4514 from a CMC patient (shown in red). The tree illustrates the phylogenetic proximity of strains 101, Evo1 and Evo2 suggesting that these 3 strains share a common ancestor and that strains Evo1 and Evo2 are likely derived from strain 101. It also illustrates the phylogenetic proximity of strains CEC4511, CEC4512, CEC4513 and CEC4514 suggesting that these 4 strains share a common ancestor and are likely derived one from another.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fróis-Martins, R., Martinez de San Vicente, K., Maufrais, C. et al. IL-17-mediated antifungal immunity restricts Candida albicans pathogenicity in the oral cavity. Nat Microbiol 11, 111–124 (2026). https://doi.org/10.1038/s41564-025-02198-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-02198-y
This article is cited by
-
Taming Candida with IL-17
Nature Microbiology (2026)