Abstract
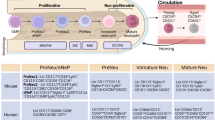
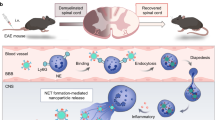
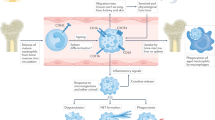
Targeted drug delivery to disease-associated activated neutrophils can provide novel therapeutic opportunities while avoiding systemic effects on immune functions. We created a nanomedicine platform that uniquely utilizes an α1-antitrypsin-derived peptide to confer binding specificity to neutrophil elastase on activated neutrophils. Surface decoration with this peptide enabled specific anchorage of nanoparticles to activated neutrophils and platelet–neutrophil aggregates, in vitro and in vivo. Nanoparticle delivery of a model drug, hydroxychloroquine, demonstrated significant reduction of neutrophil activities in vitro and a therapeutic effect on murine venous thrombosis in vivo. This innovative approach of cell-specific and activation-state-specific targeting can be applied to several neutrophil-driven pathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Modelling of docking complex with highlighted reactive centre loop and NEBP was performed using native AAT (Protein Data Bank code 1QLP)20,21. The authors declare that data supporting the findings of this study are available in their entirety within the article and its Supplementary Information. Relevant data can be provided by the corresponding authors upon reasonable request.
References
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Sorvillo, N. et al. Extracellular DNA NET-works with dire consequences for health. Circ. Res. 125, 470–488 (2019).
Albadawi, H. et al. Effect of DNase I treatment and neutrophil depletion on acute limb ischemia-reperfusion injury in mice. J. Vasc. Surg. 64, 484–493 (2016).
Ramacciotti, E. et al. P-selectin/PSGL-1 inhibitors versus enoxaparin in the resolution of venous thrombosis: a meta-analysis. Thromb. Res. 125, e138–e142 (2010).
Kim, K. et al. NOX2 is critical for heterotypic neutrophil-platelet interactions during vascular inflammation. Blood 126, 1952–1964 (2015).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 (2001).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Xu, L. et al. Heterobivalent ligands target cell-surface receptor combinations in vivo. Proc. Natl Acad. Sci. USA 109, 21295–21300 (2012).
Gunawan, R. C., Almeda, D. & Auguste, D. T. Complementary targeting of liposomes to IL-1α and TNF-α activated endothelial cells via the transient expression of VCAM1 and E-selectin. Biomaterials 32, 9848–9853 (2011).
Sapra, P. & Allen, T. M. Improved outcome when B-cell lymphoma is treated with combinations of immunoliposomal anticancer drugs targeted to both the CD19 and CD20 epitopes. Clin. Cancer Res. 10, 2530–2537 (2004).
Kanapathipillai, M., Brock, A. & Ingber, D. E. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv. Drug Deliv. Rev. 79, 107–118 (2014).
Anselmo, A. C. et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8, 11243–11253 (2014).
Wang, Z. et al. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat. Nanotechnol. 9, 204–210 (2014).
Chu, D. et al. Nanoparticle targeting of neutrophils for improved cancer immunotherapy. Adv. Healthc. Mater. 5, 1088–1093 (2016).
Robertson, J. D. et al. Targeting neutrophilic inflammation using polymersome-mediated cellular delivery. J. Immunol. 198, 3596–3604 (2017).
Liou, T. G. & Campbell, E. J. Nonisotropic enzyme–inhibitor interactions: a novel nonoxidative mechanism for quantum proteolysis by human neutrophils. Biochemistry 34, 16171–16177 (1995).
Owen, C. A. et al. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J. Cell Biol. 131, 775–789 (1995).
Stenberg, P. E. et al. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J. Cell Biol. 101, 880–886 (1985).
Elliott, P. R. et al. Inhibitory conformation of the reactive loop of α1-antitrypsin. Nat. Struct. Biol. 3, 676–681 (1996).
Elliott, P. R. et al. Topography of a 2.0 Å structure of α1-antitrypsin reveals targets for rational drug design to prevent conformational disease. Protein Sci. 9, 1274–1281 (2000).
Forsyth, S., Horvath, A. & Coughlin, P. A review and comparison of the murine α1-antitrypsin and α1-antichymotrypsin multigene clusters with the human clade A serpins. Genomics 81, 336–345 (2003).
Gehrig, S., Mall, M. A. & Schultz, C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew. Chem. Int. Ed. 51, 6258–6261 (2012).
Beatty, K., Bieth, J. & Travis, J. Kinetics of association of serine proteinases with native and oxidized α-1-proteinase inhibitor and α-1-antichymotrypsin. J. Biol. Chem. 255, 3931–3934 (1980).
Appeldoorn, C. C. et al. Rational optimization of a short human P-selectin-binding peptide leads to nanomolar affinity antagonists. J. Biol. Chem. 278, 10201–10207 (2003).
Modery, C. L. et al. Heteromultivalent liposomal nanoconstructs for enhanced targeting and shear-stable binding to active platelets for site-selective vascular drug delivery. Biomaterials 32, 9504–9514 (2011).
Daley, J. M. et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 (2008).
Elliott, J. T. & Prestwich, G. D. Maleimide-functionalized lipids that anchor polypeptides to lipid bilayers and membranes. Bioconjug. Chem. 11, 832–841 (2000).
Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 1522, 17–22 (2017).
Hope, M. J. et al. Reduction of liposome size and preparation of unilamellar vesicles by extrusion techniques. Liposome Technol. 1, 123–139 (1993).
Bennewitz, M. F. et al. Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli. JCI Insight 2, e89761 (2017).
von Bruhl, M. L. et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835 (2012).
Martinod, K. et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl Acad. Sci. USA 110, 8674–8679 (2013).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179 (2020).
Lehmann, M. et al. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI–dependent manner in an in vitro venous thrombosis model. Arterioscler. Thromb. Vasc. Biol. 38, 1052–1062 (2018).
Ramacciotti, E. et al. Evaluation of soluble P-selectin as a marker for the diagnosis of deep venous thrombosis. Clin. Appl. Thromb. Hemost. 17, 425–431 (2011).
Dyer, M. R. et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. J. Thromb. Haemost. 17, 1733–1745 (2019).
Bhattacharya, A. et al. Autophagy is required for neutrophil-mediated inflammation. Cell Rep. 12, 1731–1739 (2015).
Melles, R. B. & Marmor, M. F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 132, 1453–1460 (2014).
Mercuro, N. J. et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 9, 1036–1041 (2020).
Sames, E., Paterson, H. & Li, C. Hydroxychloroquine-induced agranulocytosis in a patient with long-term rheumatoid arthritis. Eur. J. Rheumatol. 3, 91–92 (2016).
Castanheira, F. V. S. & Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133, 2178–2185 (2019).
Silvestre-Roig, C. et al. Neutrophil diversity in health and disease. Trends Immunol. 40, 565–583 (2019).
Djie, M. Z., Stone, S. R. & Le Bonniec, B. F. Intrinsic specificity of the reactive site loop of α1-antitrypsin, α1-antichymotrypsin, antithrombin III, and protease nexin I. J. Biol. Chem. 272, 16268–16273 (1997).
Peiser, L., Mukhopadhyay, S. & Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14, 123–128 (2002).
Lahoz-Beneytez, J. et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 127, 3431–3438 (2016).
Bulbake, U. et al. Liposomal formulations in clinical use: an updated review. Pharmaceutics 9, 12 (2017).
Branzk, N. et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 (2014).
Pawlowski, C. L. et al. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials 128, 94–108 (2017).
Stavrou, E. X. et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Invest. 128, 944–959 (2018).
Stavrou, E. X. et al. Host and tumor factor XII drive ovarian cancer maintenance and progression. Blood 134, 2384–2384 (2019).
Englert, H. et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. EBioMedicine 67, 103382 (2021).
Acknowledgements
We thank the Hematopoietic Biorepository Core of Case Western Reserve University for human blood sample provision; M. Sramkoski at the Case Western Reserve University Cancer Center Flow Cytometry core facility; S. Bandyopadhyay at the Cleveland Clinic for assistance with BiaCore studies; and G. Deshpande and the Cleveland Clinic Lerner Research Institute Imaging Core for expert histologic analysis. This work was supported by the National Institutes of Health R01 HL137695 (E.X.S.); R01 HL129179, R01 HL137695, R01 HL141080, R01 HL121212 (A.S.G.); R33HL141794, R01HL120728, R01HL151984 (K.B.N.); R35 GM119526, R01 HL141080 (M.D.N.); R01 HL098217 (M.T.N.); EY022938, R24 EY024864 (T.S.K.); F32 HL149207 (J.A.); the Clinical and Translational Science Collaborative of Cleveland [UL1TR002548 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and National Institutes of Health roadmap for Medical Research (E.X.S., A.S.G.)]; Merit Review Awards (BX003851 (E.X.S.) and BX003604 (T.S.K.) from the Department of Veterans Affairs); a Case Coulter Translational Research Partnership Award (RES514649 (E.X.S., A.S.G.)); a University of Pittsburgh Physician-Scientist Award from the Burroughs Wellcome Fund (E.A.A., J.A.); a T-32 PostDoctoral Training award [NIH T32HL098036 (E.A.A.)]; an American Heart Association Scientist Development Award (E.X.S.); and the Oscar D. Ratnoff Endowed Professorship (E.X.S.). We acknowledge an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness (New York, NY; T.S.K.). S.d.M. gratefully acknowledges the Toegepaste en Technische Wetenschappen (TTW) section of the Netherlands Organization for Scientific Research (NWO, 2019/TTW/00704802). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
M.A.C., D.B., E.A.A., M.T.N., T.S.K., K.B.N., C.M., S.d.M., M.D.N., A.S.G. and E.X.S. conceptualized and planned the experiments. M.A.C., D.B., E.A.A., J.A., S.R., N.A.M., N.D.v.K., K.L.B., S.H., K. Hageman, K.M., M.S., H.L., A.B., E.M.L., K. Hart, A.G., M.d.l.F. and E.X.S. performed the experiments. M.A.C., S.d.M., A.S.G. and E.X.S. prepared the figures. M.A.C. and E.X.S. wrote the manuscript, and all authors reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
A.S.G. is a coinventor on issued patent US 9107845 (Synthetic Platelets) that is licensed from Case Western Reserve University to Haima Therapeutics. A.S.G. is a cofounder and equity stakeholder of Haima Therapeutics. The patent is on the design of a heteromultivalent NP system that can mimic the haemostatic functions of a platelet. A.S.G. is also a coinventor on issued patent US 9107963 (Heteromultivalent Nanoparticle Compositions). The patent is on the design of heteromultivalently decorated NPs for clot targeting. Although the specific NP systems described in these two patents have no direct relevance to any specific aspect of the manuscript, the context of ‘heteromultivalent NP design’ is a central aspect of the NT-NP and PNT-NP systems described in the manuscript. M.D.N. serves on the scientific advisory board of Haima Therapeutics and holds equity stake. E.X.S. is coinventor of intellectual property that has been licensed by Case Western Reserve University to XaTek and receives royalties. The patent PCT/US2017/013797 is on dielectric spectroscopy for whole blood assessment of haemostasis. This patent bears no relevance to any of the work presented in the manuscript. C.M. has been a speaker for Shire-Takeda. C.M. and S.d.M. are cofounders of TargED BV, a biotech spinout company of University Medical Center Utrecht (based upon the WO2019185723 A1 patent). C.M. and S.d.M. participate in revenue sharing as inventors through the commercialization arm of the University Medical Center Utrecht. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Benoit Ho-Tin-Noe’, Zoltan Jakus and Guillermo Ruiz-Esparza for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Videos 1 and 2 and Methods.
Supplementary Video 1
Binding of PNT-NPs to thrombus in DVT microfluidic model.
Supplementary Video 2
Binding of U-NPs to a developing thrombus in vitro.
Rights and permissions
About this article
Cite this article
Cruz, M.A., Bohinc, D., Andraska, E.A. et al. Nanomedicine platform for targeting activated neutrophils and neutrophil–platelet complexes using an α1-antitrypsin-derived peptide motif. Nat. Nanotechnol. 17, 1004–1014 (2022). https://doi.org/10.1038/s41565-022-01161-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-022-01161-w
This article is cited by
-
Engineered neutrophil membrane nanosystem for targeted siRNA therapy in myocardial ischemia–reperfusion injury
Journal of Nanobiotechnology (2025)
-
Platelet-derived biomimetic nanoplatforms: a promising drug delivery strategy for cardiovascular disease
Journal of Nanobiotechnology (2025)
-
Innovative nanoparticle-based approaches for modulating neutrophil extracellular traps in diseases: from mechanisms to therapeutics
Journal of Nanobiotechnology (2025)
-
Harnessing smart nanomaterials to reprogram neutrophil plasticity in immune modulation
Journal of Nanobiotechnology (2025)
-
Neutrophils in Cancer immunotherapy: friends or foes?
Molecular Cancer (2024)