Abstract
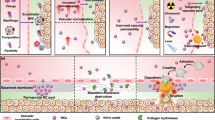
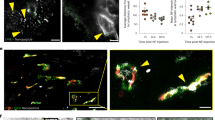
The central dogma that nanoparticle delivery to tumours requires enhanced leakiness of vasculatures is a topic of debate. To address this, we propose a single-vessel quantitative analysis method by taking advantage of protein-based nanoprobes and image-segmentation-based machine learning (nano-ISML). Using nano-ISML, >67,000 individual blood vessels from 32 tumour models were quantified, revealing highly heterogenous vascular permeability of protein-based nanoparticles. There was a >13-fold difference in the percentage of high-permeability vessels in different tumours and >100-fold penetration ability in vessels with the highest permeability compared with vessels with the lowest permeability. Our data suggest passive extravasation and transendothelial transport were the dominant mechanisms for high- and low-permeability tumour vessels, respectively. To exemplify the nano-ISML-assisted rational design of nanomedicines, genetically tailored protein nanoparticles with improved transendothelial transport in low-permeability tumours were developed. Our study delineates the heterogeneity of tumour vascular permeability and defines a direction for the rational design of next-generation anticancer nanomedicines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that data supporting the findings of this study are available within the article and its Supplementary Information. All relevant data can be made available upon reasonable request to the corresponding authors. Source data are provided with this paper.
Code availability
The source code of machine learning-based model can be accessed at https://github.com/balabilibili24/Confocal_images_analysis.git.
References
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Huang, X. et al. Hypoxia-tropic protein nanocages for modulation of tumor- and chemotherapy-associated hypoxia. ACS Nano 13, 236–247 (2019).
Dvorak, A. M. et al. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J. Leukoc. Biol. 59, 100–115 (1996).
Dvorak, A. M. & Feng, D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J. Histochem. Cytochem. 49, 419–432 (2001).
Claesson-Welsh, L. Vascular permeability—the essentials. Ups. J. Med Sci. 120, 135–143 (2015).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Butler, K. T., Davies, D. W., Cartwright, H., Isayev, O. & Walsh, A. Machine learning for molecular and materials science. Nature 559, 547–555 (2018).
Moen, E. et al. Deep learning for cellular image analysis. Nat. Methods 16, 1233–1246 (2019).
Greenwald, N. F. et al. Whole-cell segmentation of tissue images with human-level performance using large-scale data annotation and deep learning. Nat. Biotechnol. 40, 555–565 (2022).
Wei, Y. et al. Prediction and design of nanozymes using explainable machine learning. Adv. Mater. 34, e2201736 (2022).
Lin, X. et al. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 11, 814–819 (2011).
Fan, K. et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 7, 459–464 (2012).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
Caicedo, J. C. et al. Data-analysis strategies for image-based cell profiling. Nat. Methods 14, 849–863 (2017).
Falk, T. et al. U-Net: deep learning for cell counting, detection, and morphometry. Nat. Methods 16, 67–70 (2019).
Todorov, M. I. et al. Machine learning analysis of whole mouse brain vasculature. Nat. Methods 17, 442–449 (2020).
Zhang, Y. et al. Biomimetic design of mitochondria-targeted hybrid nanozymes as superoxide scavengers. Adv. Mater. 33, e2006570 (2021).
Nagy, J. A., Chang, S. H., Shih, S. C., Dvorak, A. M. & Dvorak, H. F. Heterogeneity of the tumor vasculature. Semin Thromb. Hemost. 36, 321–331 (2010).
Feng, D., Nagy, J. A., Dvorak, A. M. & Dvorak, H. F. Different pathways of macromolecule extravasation from hyperpermeable tumor vessels. Microvasc. Res. 59, 24–37 (2000).
Bonam, S. R., Wang, F. & Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 18, 923–948 (2019).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Tai, W., Li, J., Corey, E. & Gao, X. A ribonucleoprotein octamer for targeted siRNA delivery. Nat. Biomed. Eng. 2, 326–337 (2018).
Bern, M. et al. An engineered human albumin enhances half-life and transmucosal delivery when fused to protein-based biologics. Sci. Transl. Med. 12, eabb0580 (2020).
Yokota, S. & Fahimi, H. D. Immunocytochemical localization of albumin in the secretory apparatus of rat liver parenchymal cells. Proc. Natl Acad. Sci. USA 78, 4970–4974 (1981).
Shroyer, K. R. & Nakane, P. K. Immunohistochemical localization of albumin and in situ hybridization of albumin mRNA. Cell Biochem. Funct. 5, 195–210 (1987).
Pelletier, L., Jokitalo, E. & Warren, G. The effect of Golgi depletion on exocytic transport. Nat. Cell Biol. 2, 840–846 (2000).
De Matteis, M. A. & Luini, A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 9, 273–284 (2008).
Jiang, B. et al. A natural drug entry channel in the ferritin nanocage. Nano Today 35, 100948 (2020).
Huang, X. et al. Protein nanocages that penetrate airway mucus and tumor tissue. Proc. Natl Acad. Sci. USA 114, E6595–E6602 (2017).
Liu, Q. et al. Modular assembly of tumor-penetrating and oligomeric nanozyme based on intrinsically self-assembling protein nanocages. Adv. Mater. 33, e2103128 (2021).
Ronneberger, O., Fischer, P. & Brox, T. U-Net: convolutional networks for biomedical image segmentation. Med. Image Comput. Comput. Assist. Interv. 9351, 234–241 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China 91959129 (X.H.), 32271448 (X.H.), 82072054 (J.Z.), 31870999 (X.H.), the National Key Research and Development Program of China 2022YFA1105100 (X.H.), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project TSBICIP-KJGG-014-03 (X.H.) and the Nankai University Hundred Young Academic Leaders Program.
Author information
Authors and Affiliations
Contributions
M.Z., J.Z. and X.H. conceived the idea, collected data, conducted data analysis and performed all the experiments. Z.L. assisted with the development of the U-net model. M.Z. and X.H extracted data information from U-net. Q.L., Z.G., Z.Z., T.Q., J.T. and R.Z. performed cryosections and immunostaining. A.C.M. provided guidance and edited the manuscript. D.K., J.T. and X.Y. helped to guide part of experiments. X.H. designed and supervised all studies and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Bjoern Menze, Constantinos Mikelis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Tables 1–5.
Source data
Source Data Fig. 2
Statistical source data
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Unprocessed gels and statistical source data
Source Data Fig. 5
Statistical source data
Source Data Fig. 6
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, M., Zhuang, J., Li, Z. et al. Machine-learning-assisted single-vessel analysis of nanoparticle permeability in tumour vasculatures. Nat. Nanotechnol. 18, 657–666 (2023). https://doi.org/10.1038/s41565-023-01323-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-023-01323-4
This article is cited by
-
Granzyme B-mimetic nanozyme for nanovesicle targeted anticancer applications
Nature Communications (2026)
-
Applications of liposomes and lipid nanoparticles in cancer therapy: current advances and prospects
Experimental Hematology & Oncology (2025)
-
Recent advances of engineering cell membranes for nanomedicine delivery across the blood–brain barrier
Journal of Nanobiotechnology (2025)
-
Nanoparticle-based strategy in CAR-T cell immunotherapy: challenges, implications, and perspectives
Molecular Cancer (2025)
-
SynBioNanoDesign: pioneering targeted drug delivery with engineered nanomaterials
Journal of Nanobiotechnology (2025)