Abstract
Nerve–cancer crosstalk has gained substantial attention owing to its impact on tumour growth, metastasis and therapy resistance. Effective therapeutic strategies targeting tumour-associated nerves within the intricate tumour microenvironment remain a major challenge in pancreatic cancer. Here we develop Escherichia coli Nissle 1917-derived outer membrane vesicles conjugated with nerve-binding peptide NP41, loaded with the tropomyosin receptor kinase (Trk) inhibitor larotrectinib (Lar@NP-OMVs) for tumour-associated nerve targeting. Lar@NP-OMVs achieve efficient nerve intervention to diminish neurite growth by disrupting the neurotrophin/Trk signalling pathway. Moreover, OMV-mediated repolarization of M2-like tumour-associated macrophages to an M1-like phenotype results in nerve injury, further accentuating Lar@NP-OMV-induced nerve intervention to inhibit nerve-triggered proliferation and migration of pancreatic cancer cells and angiogenesis. Leveraging this strategy, Lar@NP-OMVs significantly reduce nerve infiltration and neurite growth promoted by gemcitabine within the tumour microenvironment, leading to augmented chemotherapy efficacy in pancreatic cancer. This study sheds light on a potential avenue for nerve-targeted therapeutic intervention for enhancing pancreatic cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings in this study are available within this article and its Supplementary Information. The raw transcriptome data used in this paper were deposited in the NCBI Sequence Read Archive under accession number PRJNA1082402. Due to the very large file size and volume of data, the remaining raw data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. Pancreatic cancer. Lancet 395, 2008–2020 (2020).
Neoptolemos, J. P. et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 15, 333–348 (2018).
Wood, L. D., Canto, M. I., Jaffee, E. M. & Simeone, D. M. Pancreatic cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology 163, 386–402 (2022).
Binenbaum, Y., Na’ara, S. & Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updat. 23, 55–68 (2015).
Ho, W. J., Jaffee, E. M. & Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 17, 527–540 (2020).
Sherman, M. H. & Beatty, G. L. Tumor microenvironment in pancreatic cancer pathogenesis and therapeutic resistance. Annu. Rev. Pathol. 18, 123–148 (2023).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Amit, M. et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454 (2020).
Zhang, Y. et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 34, 1999–2017 (2022).
Banh, R. S. et al. Neurons release serine to support mRNA translation in pancreatic cancer. Cell 183, 1202–1218 (2020).
Renz, B. W. et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90 (2018).
Demir, I. E., Friess, H. & Ceyhan, G. O. Neural plasticity in pancreatitis and pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 12, 649–659 (2015).
Hanahan, D. & Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 41, 573–580 (2023).
Jurcak, N. R. et al. Axon guidance molecules promote perineural invasion and metastasis of orthotopic pancreatic tumors in mice. Gastroenterology 157, 838–850 (2019).
Deshpande, K. et al. Neuronal exposure induces neurotransmitter signaling and synaptic mediators in tumors early in brain metastasis. Neuro Oncol. 24, 914–924 (2022).
Cervantes-Villagrana, R. D., Albores-García, D., Cervantes-Villagrana, A. R. & García-Acevez, S. J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target. Ther. 5, 99 (2020).
Khanmammadova, N., Islam, S., Sharma, P. & Amit, M. Neuro-immune interactions and immuno-oncology. Trends Cancer 9, 636–649 (2023).
Li, J., Kang, R. & Tang, D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun. 41, 642–660 (2021).
Sugimoto, M. et al. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur. J. Cancer 50, 1900–1908 (2014).
Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017).
Chang, A. et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 15, eadf1147 (2023).
Huang, E. J. & Reichardt, L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003).
Nakagawara, A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 169, 107–114 (2001).
O’Keeffe, G. W., Gutierrez, H., Pandolfi, P. P., Riccardi, C. & Davies, A. M. NGF-promoted axon growth and target innervation requires GITRL-GITR signaling. Nat. Neurosci. 11, 135–142 (2008).
Silverman, D. A. et al. Cancer-associated neurogenesis and nerve–cancer cross-talk. Cancer Res. 81, 1431–1440 (2021).
Allen, J. K. et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 78, 3233–3242 (2018).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540 (2020).
Liu, D. et al. Characterization of on-target adverse events caused by TRK inhibitor therapy. Ann. Oncol. 31, 1207–1215 (2020).
Jahromi, L. P. & Fuhrmann, G. Bacterial extracellular vesicles: understanding biology promotes applications as nanopharmaceuticals. Adv. Drug Deliv. Rev. 173, 125–140 (2021).
Li, M. et al. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J. Control. Release 323, 253–268 (2020).
Zhuang, W. R. et al. Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity. Nat. Commun. 14, 1675 (2023).
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M. & Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 21, 415–430 (2023).
Wei, B. et al. Polarization of tumor-associated macrophages by nanoparticle-loaded Escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett. 21, 4231–4240 (2021).
Qin, J. et al. Bacterial outer membrane vesicle-templated biomimetic nanoparticles for synergistic photothermo-immunotherapy. Nano Today 46, 101591 (2022).
Puurunen, M. K. et al. Safety and pharmacodynamics of an engineered E. coli Nissle for the treatment of phenylketonuria: a first-in-human phase 1/2a study. Nat. Metab. 3, 1125–1132 (2021).
Whitney, M. A. et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat. Biotechnol. 29, 352–356 (2011).
You, H. et al. Sight and switch off: nerve density visualization for interventions targeting nerves. Sci. Adv. 6, eaax6040 (2020).
Kaduri, M. et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases. Sci. Adv. 7, eabj5435 (2021).
Madeo, M. et al. Cancer exosomes induce tumor innervation. Nat. Commun. 9, 4284 (2018).
Tian, Z. et al. TIMP1 derived from pancreatic cancer cells stimulates Schwann cells and promotes the occurrence of perineural invasion. Cancer Lett. 546, 215863 (2022).
Gysler, S. M. & Drapkin, R. Tumor innervation: peripheral nerves take control of the tumor microenvironment. J. Clin. Invest. 131, e147276 (2021).
Arnaoutova, I. & Kleinman, H. K. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 5, 628–635 (2010).
Feng, Q. et al. Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv. Mater. 34, 2206200 (2022).
Borsini, A., Zunszain, P. A., Thuret, S. & Pariante, C. M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 38, 145–157 (2015).
Neumann, H. et al. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J. Neurosci. 22, 854–862 (2002).
Wei, Z. et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 12, 440 (2021).
Chu, X. et al. Blocking cancer–nerve crosstalk for treatment of metastatic bone cancer pain. Adv. Mater. 34, 2108653 (2022).
Malin, S. A., Davis, B. M. & Molliver, D. C. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2, 152–160 (2007).
Martinez-Jothar, L. et al. Insights into maleimide-thiol conjugation chemistry: conditions for efficient surface functionalization of nanoparticles for receptor targeting. J. Control. Release 282, 101–109 (2018).
Wang, Z. et al. Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy. Nat. Nanotechnol. 16, 1130–1140 (2021).
Acknowledgements
This work was supported by National Key R&D Program of China (2022YFA1206000 to T.Y., 2020YFA0710700 to L.G., 2021YFA1201200 to T.Y. and 2022YFA1206100 to X.Y.), National Natural Science Foundation of China (82272844 to L.G., 82073796 and 81627901 to X.Y., 82330060 and 92359304 to S.J.) and Program for HUST Academic Frontier Youth Team (2018QYTD01 to L.G.). We thank J. Zhao (Huazhong University of Science and Technology, Wuhan, China) for providing the KPC cells. We thank the Research Core Facilities for Life Science (HUST), Optical Bioimaging Core Facility of WNLO-HUST and the Analytical and Testing Center of Huazhong University of Science and Technology for related analysis.
Author information
Authors and Affiliations
Contributions
L.G., X.Y. and J.Q. designed the project. J.Q., J. Liu, Z.W., X.L., Z.C., J. Li, W.Z., H.L., S.X., T.Y. and B.Z performed the experiments. J.Q., J. Liu, S.G., S.J., G.-J.T., X.Y. and L.G. analysed and interpreted the data. J.Q. and L.G. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of Lar@NP-OMVs.
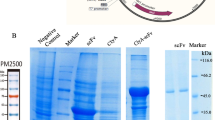
a Morphology of OMVs, NP-OMVs, Lar@OMVs and Lar@NP-OMVs by TEM. Scale bar, 100 nm. Images are representative of three independent samples. b,c Size distribution (b) and zeta potential (c) of OMVs, NP-OMVs, Lar@OMVs and Lar@NP-OMVs by NTA and DLS analysis, respectively. Data are presented as means ± s.d. (n = 3 independent samples). d In vitro Lar release of Lar@OMVs and Lar@NP-OMVs in PBS at different pH values. Data are presented as means ± s.d. (n = 3 independent samples). e,f Hydrodynamic diameter (e) and zeta potential (f) of OMVs, NP-OMVs, Lar@OMVs and Lar@NP-OMVs in PBS with 10% mouse serum for different time intervals by DLS analysis. Data are presented as means ± s.d. (n = 3 independent samples).
Extended Data Fig. 2 Nerve targeting capability of Lar@MP-OMVs.
a DiD mean fluorescence intensity (MFI) in PC12 cells, DRG neural cells, Panc02 cells and HUVECs after treatment with DiD-labeled OMVs or NP-OMVs for 6 h. Data are presented as means ± s.d. (n = 4 biologically independent samples). b Relative Lar content in PC12 cells, DRG neural cells, Panc02 cells and HUVECs after treatment with free Lar, Lar@OMVs or Lar@NP-OMVs for 6 h by HPLC. Data are presented as means ± s.d. (n = 3 biologically independent samples). c,d In vivo representative IR780 imaging (c) and fluorescence intensity (d) of tumours in orthotopic Panc02 tumour-bearing mice at different time intervals after intravenous injection of IR780-labeled OMVs or NP-OMVs. Data are presented as means ± s.d. for d (n = 3 mice per group). e,f Ex vivo IR780 imaging (e) and fluorescence intensity (f) of the major organs and tumours of orthotopic Panc02 tumour-bearing mice at 72 h after treatment indicated in c. Data are presented as means ± s.d. for f (n = 3 mice per group). g,h Representative images (g) and quantification (h) of the co-localization of IR780-labeled OMVs or NP-OMVs with β3-tubulin-labeled nerves in tumour tissues of orthotopic Panc02 tumour-bearing mice at 72 h after treatment indicated in c. Images are representative of three independent samples. Scale bars, 50 µm for g. Data are presented as means ± s.d. for h (n = 12 fields in total from 3 mice). The P values in a, b, d and f were determined using two-way ANOVA followed by Tukey’s multiple comparisons test. The P value in h was determined using unpaired two-tailed Student's t-test.
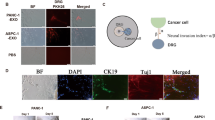
Extended Data Fig. 3 Efficient inhibition of tumour cell proliferation and improved tumour microenvironment by combination of GEM and Lar@NP-OMVs in fresh tumour slices from pancreatic cancer patient.
a,c Representative immunofluorescence images of GAP43 (a) and β3-tubulin (c) in slices from pancreatic cancer patient-derived tumour after treatment with PBS, GEM, Lar@NP-OMVs or combination of GEM and Lar@NP-OMVs for 4 days. The cell nuclei were stained with DAPI (blue). Scale bars, 50 µm. b,d Quantification of GAP43+ (b) and β3-tubulin+ (d) areas in tumour slices from pancreatic cancer patient after treatment indicated in a. Data are presented as means ± s.d. (n = 10 fields in total from 3 biologically independent samples). e-g Percentages of M1-like TAMs (e), M2-like TAMs (f) and proliferating tumour cells (g) in tumour slices from pancreatic cancer patient after treatment indicated in a by flow cytometry. Data are presented as means ± s. d. (n = 3 biologically independent samples). h,i Representative immunofluorescence images (h) and numbers (i) of MUC1+Ki67+ cells in tumour slices from pancreatic cancer patient after treatment indicated in a. The tumour slices were stained with anti-MUC1 antibody (green) and anti-Ki67 antibody (red). The cell nuclei were stained with DAPI (blue). Scale bars, 50 µm for h. Data are presented as means ± s.d. for i (n = 6 fields in total from 3 biologically independent samples). Images in a, c and h are representative of three independent experiments. The P values in b, d-g and i were determined using one-way ANOVA followed by Tukey’s multiple comparisons test.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–54 and Table 1.
Supplementary Data
Source data for Supplementary figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, J., Liu, J., Wei, Z. et al. Targeted intervention in nerve–cancer crosstalk enhances pancreatic cancer chemotherapy. Nat. Nanotechnol. 20, 311–324 (2025). https://doi.org/10.1038/s41565-024-01803-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-024-01803-1
This article is cited by
-
Hijacking homeostasis: the brain-body neural circuitry in tumor pathogenesis and emerging therapeutic frontiers
Molecular Cancer (2025)
-
Sympathetic nervous system in tumor progression and metabolic regulation: mechanisms and clinical potential
Journal of Translational Medicine (2025)
-
Reprogramming neural-tumor crosstalk: emerging therapeutic dimensions and targeting strategies
Military Medical Research (2025)
-
Cancer neuroscience in head and neck: interactions, modulation, and therapeutic strategies
Molecular Cancer (2025)
-
Micro/nano motors treating of digestive system diseases
Journal of Nanobiotechnology (2025)