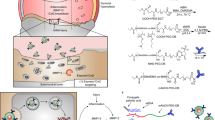
Abstract
Intra-articular RNA therapeutics have shown promise in osteoarthritis (OA); however, maximizing their efficacy requires targeted delivery to degenerating cartilage within focal lesions. As OA progresses, cartilage degeneration worsens, necessitating disease-responsive targeting with enhanced delivery in advanced stages. Here we develop an anionic nanoparticle (NP) strategy for targeting glycosaminoglycan loss, a hallmark of OA’s progression that reduces cartilage’s negative charge. These NPs selectively diffuse and accumulate into matrix regions inversely correlated with glycosaminoglycan content owing to reduced electrostatic repulsion, a strategy we term ‘matrix inverse targeting’ (MINT). In a mouse model of OA, intra-articular delivery of luciferase messenger RNA-loaded MINT NPs demonstrated disease-severity-responsive expression. Using this strategy, we delivered ghrelin mRNA, as ghrelin has shown chondroprotection properties previously. Ghrelin mRNA-loaded MINT NPs reduced cartilage degeneration, subchondral bone thickening and nociceptive pain. Our findings highlight the potential of ghrelin mRNA delivery as a disease-modifying therapy for OA and the platform’s potential for lesion-targeted RNA delivery responsive to disease severity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data that support the findings of this study are available in the paper and the Supplementary Information. Source/raw data will be available for research purposes from the corresponding authors upon reasonable request.
References
Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325, 568–578 (2021).
Jiang, P., Hu, K., Jin, L. & Luo, Z. A brief review of current treatment options for osteoarthritis including disease-modifying osteoarthritis drugs (DMOADs) and novel therapeutics. Ann. Med. Surg. 86, 4042–4048 (2024).
Li, X., Shen, L., Deng, Z. & Huang, Z. New treatment for osteoarthritis: gene therapy. Precis. Clin. Med. 6, pbad014 (2023).
Evans, C. H., Ghivizzani, S. C. & Robbins, P. D. Osteoarthritis gene therapy in 2022. Curr. Opin. Rheumatol. 35, 37–43 (2023).
Bedingfield, S. K. et al. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 5, 1069–1083 (2021).
Nap, R. J. & Szleifer, I. Structure and interactions of aggrecans: statistical thermodynamic approach. Biophys. J. 95, 4570–4583 (2008).
Bittersohl, B. et al. Delayed gadolinium-enhanced magnetic resonance imaging of hip joint cartilage: pearls and pitfalls. Orthop. Rev. 3, e11 (2011).
Tiderius, C. J., Olsson, L. E., Leander, P., Ekberg, O. & Dahlberg, L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn. Reson. Med. 49, 488–492 (2003).
Xiao, S., Tang, Y., Lin, Y., Lv, Z. & Chen, L. Tracking osteoarthritis progress through cationic nanoprobe-enhanced photoacoustic imaging of cartilage. Acta Biomater. 109, 153–162 (2020).
Chen, L. et al. Cationic poly-l-lysine-encapsulated melanin nanoparticles as efficient photoacoustic agents targeting to glycosaminoglycans for the early diagnosis of articular cartilage degeneration in osteoarthritis. Nanoscale 10, 13471–13484 (2018).
Lu, R. et al. Gadolinium-hyaluronic acid nanoparticles as an efficient and safe magnetic resonance imaging contrast agent for articular cartilage injury detection. Bioact. Mater. 5, 758–767 (2020).
Fazeli, P. K. et al. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. J. Clin. Psychiatry 79, 17m11585 (2018).
Tschop, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Guillory, B. et al. Deletion of ghrelin prevents aging-associated obesity and muscle dysfunction without affecting longevity. Aging Cell 16, 859–869 (2017).
Baatar, D., Patel, K. & Taub, D. D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 340, 44–58 (2011).
Andrews, Z. B. et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454, 846–851 (2008).
Stoyanova, I. I. Ghrelin: a link between ageing, metabolism and neurodegenerative disorders. Neurobiol. Dis. 72 Pt A, 72–83 (2014).
Sibilia, V. et al. Pharmacological characterization of the ghrelin receptor mediating its inhibitory action on inflammatory pain in rats. Amino Acids 43, 1751–1759 (2012).
Sun, J. et al. Role and molecular mechanism of ghrelin in degenerative musculoskeletal disorders. J. Cell. Mol. Med. 27, 3681–3691 (2023).
Liu, J. et al. Ghrelin prevents articular cartilage matrix destruction in human chondrocytes. Biomed. Pharmacother. 98, 651–655 (2018).
Qu, R. et al. Ghrelin protects against osteoarthritis through interplay with Akt and NF-kappaB signaling pathways. FASEB J. 32, 1044–1058 (2018).
Bautista, C. A., Park, H. J., Mazur, C. M., Aaron, R. K. & Bilgen, B. Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PLoS ONE 11, e0158976 (2016).
Bergholt, N. L., Lysdahl, H., Lind, M. & Foldager, C. B. A standardized method of applying toluidine blue metachromatic staining for assessment of chondrogenesis. Cartilage 10, 370–374 (2019).
Bajpayee, A. G. & Grodzinsky, A. J. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat. Rev. Rheumatol. 13, 183–193 (2017).
Gao, T. et al. Non-destructive spatial mapping of glycosaminoglycan loss in native and degraded articular cartilage using confocal Raman microspectroscopy. Front. Bioeng. Biotechnol. 9, 744197 (2021).
Pauli, C. et al. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis Cartilage 20, 476–485 (2012).
Kleuskens, M. W. A., van Donkelaar, C. C., Kock, L. M., Janssen, R. P. A. & Ito, K. An ex vivo human osteochondral culture model. J. Orthop. Res. 39, 871–879 (2021).
Wei, Y. et al. Phospholipase A2 inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Sci. Adv. 7, eabe6374 (2021).
Abumanhal-Masarweh, H. et al. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J. Control. Release 307, 331–341 (2019).
Uchimura, T. et al. Erythromycin acts through the ghrelin receptor to attenuate inflammatory responses in chondrocytes and maintain joint integrity. Biochem. Pharmacol. 165, 79–90 (2019).
Drevet, S., Favier, B., Brun, E., Gavazzi, G. & Lardy, B. Mouse models of osteoarthritis: a summary of models and outcomes assessment. Comp. Med. 72, 3–13 (2022).
Yu, L. et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol. Pain 4, 61 (2008).
DeJulius, C. R. et al. Engineering approaches for RNA-based and cell-based osteoarthritis therapies. Nat. Rev. Rheumatol. 20, 81–100 (2024).
Gao, J. et al. Gene therapy for CNS disorders: modalities, delivery and translational challenges. Nat. Rev. Neurosci. 25, 553–572 (2024).
Chen, H. et al. Urchin-like ceria nanoparticles for enhanced gene therapy of osteoarthritis. Sci. Adv. 9, eadf0988 (2023).
Shin, H. J. et al. p47phox siRNA-loaded PLGA nanoparticles suppress ROS/oxidative stress-induced chondrocyte damage in osteoarthritis. Polymers 12, 443 (2020).
Aini, H. et al. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci. Rep. 6, 18743 (2016).
Yan, H. et al. Induction of WNT16 via peptide-mRNA nanoparticle-based delivery maintains cartilage homeostasis. Pharmaceutics 12, 73 (2020).
Geiger, B. C., Wang, S., Padera, R. F. Jr., Grodzinsky, A. J. & Hammond, P. T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 10, eaat8800 (2018).
Vedadghavami, A. et al. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater. 93, 258–269 (2019).
Konttinen, Y. T. et al. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 46, 953–960 (2002).
Paulini, F. et al. In vivo evaluation of DMSA-coated magnetic nanoparticle toxicity and biodistribution in rats: a long-term follow-up. Nanomaterials 12, 3513 (2022).
Dantas, G. P. F., Ferraz, F. S., Andrade, L. M. & Costa, G. M. J. Male reproductive toxicity of inorganic nanoparticles in rodent models: a systematic review. Chem. Biol. Interact. 363, 110023 (2022).
Li, Y., Vulpe, C., Lammers, T. & Pallares, R. M. Assessing inorganic nanoparticle toxicity through omics approaches. Nanoscale 16, 15928–15945 (2024).
Akalu, Y., Molla, M. D., Dessie, G. & Ayelign, B. Physiological effect of ghrelin on body systems. Int. J. Endocrinol. 2020, 1385138 (2020).
Wakabayashi, H., Arai, H. & Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J. Cachexia Sarcopenia Muscle 12, 14–16 (2021).
Yasar, H. et al. Kinetics of mRNA delivery and protein translation in dendritic cells using lipid-coated PLGA nanoparticles. J. Nanobiotechnol. 16, 72 (2018).
Lutz, J. et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. npj Vaccines 2, 29 (2017).
Pardi, N. et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 217, 345–351 (2015).
Qin, S. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 7, 166 (2022).
Unti, M. J. & Jaffrey, S. R. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem. Biol. 31, 163–176 e165 (2024).
Musumeci, G. et al. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur. J. Histochem. 58, 2371 (2014).
Zhang, L. et al. Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2, 1696–1702 (2008).
Xiao, Y. et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 13, 758 (2022).
Kong, N. et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 11, eaaw1565 (2019).
Huang, X. et al. Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 17, 748–780 (2022).
Uchimura, T., Foote, A. T., Smith, E. L., Matzkin, E. G. & Zeng, L. Insulin-like growth factor II (IGF-II) inhibits IL-1beta-induced cartilage matrix loss and promotes cartilage integrity in experimental osteoarthritis. J. Cell. Biochem. 116, 2858–2869 (2015).
Kawamoto, T. & Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamoto’s film method (2020). Methods Mol. Biol. 2230, 259–281 (2021).
Glasson, S. S., Blanchet, T. J. & Morris, E. A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15, 1061–1069 (2007).
Sleigh, J. N., Weir, G. A. & Schiavo, G. A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res. Notes 9, 82 (2016).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Kameda, T., Kaneuchi, Y., Sekiguchi, M. & Konno, S. I. Measurement of mechanical withdrawal thresholds and gait analysis using the CatWalk method in a nucleus pulposus-applied rodent model. J. Exp. Orthop. 4, 31 (2017).
Kawamoto, T. & Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot’s film method (2012). Methods Mol. Biol. 1130, 149–164 (2014).
McNulty, M. A. et al. A comprehensive histological assessment of osteoarthritis lesions in mice. Cartilage 2, 354–363 (2011).
Krenn, V. et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49, 358–364 (2006).
Acknowledgements
This work was supported by funding from Ellison Foundation (to L.Z. and N.J.), Prime Minister Research Fellowship (PMRF) (to M.D.), NIH grant 1R01AR077718 (to N.J.), 1R01AR077146-01A1 (L.Z.) and 1R21AR085398-01 (to L.Z. and N.J.). The work of S. Lee was supported by the Nano and Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (RS-2024-00405574). S.D.K. acknowledges the support received by the ‘Khorana Program for Scholars’ awarded by the Indo-US Science and Technology Forum (IUSSTF). We utilized ChatGPT (OpenAI) to aid in language refinement and improve the clarity and cohesiveness of this paper. All intellectual content, scientific interpretations and conclusions remain the sole responsibility of the authors.
Author information
Authors and Affiliations
Contributions
M.D., A.R.M., J.G., L.Z. and N.J. conceived and designed the project. M.D., A.R.M., M.R., N.B., S. Liu, J.L., J.G., N.P., E.B., A.B., C.J., A.G., S.D.K., K.C., A.N., J.K. and Z.X. performed the experiments and analysed the data. D.P. conducted and analysed the molecular docking simulation studies. M.D., A.R.M., N.J. and L.Z. wrote the paper. J.G., S. Lee and J.M.K. edited the paper. N.J. and L.Z. supervised the overall research. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
N.J., J.M.K., L.Z., M.D., J.G. and A.R.M. have one pending patent (US patent application number 63/756,779) based on the nanoparticle technology described in this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Christopher Evans and Fergal O’Brien for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Methods, Tables 1 and 2, Figs. 1–7 and sequence of ghrelin mRNA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dewani, M., Mamidwar, A.R., Rawal, M. et al. A disease-severity-responsive nanoparticle enables potent ghrelin messenger RNA therapy in osteoarthritis. Nat. Nanotechnol. (2026). https://doi.org/10.1038/s41565-025-02101-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41565-025-02101-0
This article is cited by
-
Lesion-targeted, severity-responsive nanoparticle delivery for RNA therapy in osteoarthritis
Nature Nanotechnology (2026)