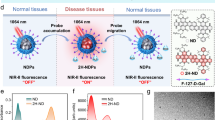
Abstract
Cancer imaging approaching single-cell levels is highly desirable for studying in vivo cell migration and cancer metastasis. However, current imaging probes struggle to simultaneously achieve high sensitivity, deep-tissue penetration and tissue specificity. Here we report size- and shape-resolved fluorescent DNA framework (FDF) dots with tail emission in the second near-infrared window (1,000–1,700 nm, NIR-II), which enable near-single-cell-level, tumour-targeting deep-tissue (~1 cm) NIR-II imaging in tumour-bearing mouse models. The construction of DNA frameworks with embedded hydrophobic nanocavity results in the non-covalent encapsulation of a designed NIR-Ib (900–1,000 nm) probe (dye Sq964). The FDF dots exhibit high water solubility, brightness and photostability. We find that the stable tumour retention of FDF dots with enhanced signal intensity arises from their shape-dependent accumulation in tumour cells. FDF-dot-based cancer imaging reveals in vivo sensitivity down to ~40 tumour cells, high tumour-to-normal tissue ratios up to ~26 and long-term imaging over 11 days. We also demonstrate NIR-II-image-guided breast cancer surgery with the complete excision of metastases with a minimum size of ~53 μm (~20 cells).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information. Additional data and files are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Jafari, S. H. et al. Breast cancer diagnosis: imaging techniques and biochemical markers. J. Cell. Physiol. 233, 5200–5213 (2018).
Wang, F. et al. High-precision tumor resection down to few-cell level guided by NIR-IIb molecular fluorescence imaging. Proc. Natl Acad. Sci. USA 119, e2123111119 (2022).
Sheng, Z. et al. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumors. Adv. Mater. 30, 1800766 (2018).
Zhu, W. et al. Zwitterionic AIEgens: rational molecular design for NIR-II fluorescence imaging-guided synergistic phototherapy. Adv. Funct. Mater. 31, 2007026 (2020).
Lu, L. F. et al. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 11, 4192 (2020).
Ling, S. S. et al. Tumor microenvironment-activated NIR-II nanotheranostic system for precise diagnosis and treatment of peritoneal metastasis. Angew. Chem. Int. Ed. 59, 7219–7223 (2020).
Wang, T. et al. A hybrid erbium(iii)-bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nat. Mater. 20, 1571–1578 (2021).
Liu, Y. et al. Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: from strategic design toward molecular imaging and theranostics. Chem. Rev. 122, 209–268 (2022).
Wang, P. et al. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 9, 2898 (2018).
Wang, S. et al. In vivo high-resolution ratiometric fluorescence imaging of inflammation using NIR-II nanoprobes with 1550 nm emission. Nano Lett. 19, 2418–2427 (2019).
Wang, R., Zhou, L., Wang, W. X., Li, X. M. & Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 8, 14702 (2017).
Santos, H. D. A. et al. Ultrafast photochemistry produces superbright short-wave infrared dots for low-dose in vivo imaging. Nat. Commun. 11, 2933 (2020).
Wan, H. et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 9, 1171 (2018).
Li, B. et al. Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging. Nat. Commun. 11, 3102 (2020).
Antaris, A. L. et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 15, 235–242 (2016).
Li, Y. et al. Small-molecule fluorophores for near-infrared IIb imaging and image-guided therapy of vascular diseases. CCS Chem. 4, 3735–3750 (2022).
Yang, Q. L. et al. Donor engineering for NIR-II molecular fluorophores with enhanced fluorescent performance. J. Am. Chem. Soc. 140, 1715–1724 (2018).
Antaris, A. L. et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 8, 15269 (2017).
Kenry, Duan, Y. & Liu, B. Recent advances of optical imaging in the second near-infrared window. Adv. Mater. 30, 1802394 (2018).
Niekamp, S., Stuurman, N. & Vale, R. D. A 6-nm ultra-photostable DNA FluoroCube for fluorescence imaging. Nat. Methods 17, 437–441 (2020).
Liang, L. et al. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew. Chem. Int. Ed. 53, 7745–7750 (2014).
Edwardson, T. G. W., Carneiro, K. M. M., McLaughlin, C. K., Serpell, C. J. & Sleiman, H. F. Site-specific positioning of dendritic alkyl chains on DNA cages enables their geometry-dependent self-assembly. Nat. Chem. 5, 868–875 (2013).
Ding, H. et al. DNA nanostructure-programmed like-charge attraction at the cell-membrane interface. ACS Cent. Sci. 4, 1344–1351 (2018).
Bastings, M. M. C. et al. Modulation of the cellular uptake of DNA origami through control over mass and shape. Nano Lett. 18, 3557–3564 (2018).
Wang, P. et al. Visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells. J. Am. Chem. Soc. 140, 2478–2484 (2018).
Chou, L. Y., Zagorovsky, K. & Chan, W. C. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat. Nanotechnol. 9, 148–155 (2014).
Ge, Z., Gu, H., Li, Q. & Fan, C. Concept and development of framework nucleic acids. J. Am. Chem. Soc. 140, 17808–17819 (2018).
Wiraja, C. et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat. Commun. 10, 1147 (2019).
Peng, X. et al. DNA nanostructure-programmed cell entry via corner angle-mediated molecular interaction with membrane receptors. Nano Lett. 21, 6946–6951 (2021).
Cosco, E. D. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020).
Liu, D. K. et al. Xanthene-based NIR-II dyes for in vivo dynamic imaging of blood circulation. J. Am. Chem. Soc. 143, 17136–17143 (2021).
Xu, W., Wang, D. & Tang, B. Z. NIR-II AIEgens: a win-win integration towards bioapplications. Angew. Chem. Int. Ed. 60, 7476–7487 (2021).
Zhang, Q. et al. Anti-inflammatory and antioxidative effects of tetrahedral DNA nanostructures via the modulation of macrophage responses. ACS Appl. Mater. Interfaces 10, 3421–3430 (2018).
Du, R. R. et al. Innate immune stimulation using 3D wireframe DNA origami. ACS Nano 16, 20340–20352 (2022).
Lucas, C. R. et al. DNA origami nanostructures elicit dose-dependent immunogenicity and are nontoxic up to high doses in vivo. Small 18, e2108063 (2022).
Lu, J. Q. et al. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano 12, 11041–11061 (2018).
Paschall, A. V. & Liu, K. B. An orthotopic mouse model of spontaneous breast cancer metastasis. J. Vis. Exp. 114, 54040 (2016).
Wojtynek, N. E. et al. Nanoparticle formulation of indocyanine green improves image-guided surgery in a murine model of breast cancer. Mol. Imaging Biol. 22, 891–903 (2020).
Speak, A. O. et al. A high-throughput in vivo screening method in the mouse for identifying regulators of metastatic colonization. Nat. Protoc. 12, 2465–2477 (2017).
Goldman, E. et al. Nanoparticles target early-stage breast cancer metastasis in vivo. Nanotechnology 28, 43LT01 (2017).
Lou, Y. et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 71, 3364–3376 (2011).
Sun, H. et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv. Mater. 28, 9581–9588 (2016).
Gao, H. et al. Far-red/near-infrared emissive (1,3-dimethyl)barbituric acid-based AIEgens for high-contrast detection of metastatic tumors in the lung. Chem. Asian J. 14, 871–876 (2019).
Kawakita, N., Takizawa, H., Kondo, K., Sakiyama, S. & Tangoku, A. Indocyanine green fluorescence navigation thoracoscopic metastasectomy for pulmonary metastasis of hepatocellular carcinoma. Ann. Thorac. Cardiovasc. Surg. 22, 367–369 (2016).
Keating, J. et al. Near-infrared intraoperative molecular imaging can locate metastases to the lung. Ann. Thorac. Surg. 103, 390–398 (2017).
On, K. C. et al. Tumor-targeting glycol chitosan nanoparticles for image-guided surgery of rabbit orthotopic VX2 lung cancer. Pharmaceutics 12, 621 (2020).
Wada, H. et al. Intraoperative near-infrared fluorescence-guided peripheral lung tumor localization in rabbit models. Ann. Thorac. Surg. 107, 248–256 (2019).
Zhou, H. et al. Specific small-molecule NIR-II fluorescence imaging of osteosarcoma and lung metastasis. Adv. Healthc. Mater. 9, e1901224 (2020).
Mao, Y. et al. The identification of sub-centimetre nodules by near-infrared fluorescence thoracoscopic systems in pulmonary resection surgeries. Eur. J. Cardiothorac. Surg. 52, 1190–1196 (2017).
Dang, X. et al. Deep-tissue optical imaging of near cellular-sized features. Sci. Rep. 9, 3873 (2019).
Zhao, Y. et al. A temporally resolved DNA framework state machine in living cells. Nat. Mach. Intell. 5, 980–990 (2023).
Acknowledgements
We thank F. Zhang (Fudan University) and Q. Wang (Suzhou Institute of Nano-Tech and Nano-Bionics) for the NIR imaging assistance. This work was supported by the National Key R&D Program of China (2023YFC3404200 to Y.Z.), the National Natural Science Foundation of China (T2188102 to C.F., 21991134 to C.F., 22325406 to J.L. and 12305400 to Q.Y.), 2022 Shanghai ‘Science and Technology Innovation Action Plan’ Fundamental Research Project (22JC1401203 to Y.Z.) and the Xiangfu Lab Research Project (XF012022E0100 to C.F.). We would like to dedicate this paper to C.Z., who unfortunately passed away just before this paper was revised. C.Z. played an essential role in the research described here and he is greatly missed.
Author information
Authors and Affiliations
Contributions
C.F. and Y.Z. directed the research. C.F., Y.Z., J.L., C.Z., Xia Liu and B.S. conceived and designed the experiments. Xia Liu, B.S., Y.G., S.Z., Q.Y. and Xiaoguo Liu carried out the experiments and analysed the data. J.S., Q.L., L.W., J.L., H.T. and I.W. provided suggestions and technical support on the project. J.L., Y.Z. and C.F. wrote and revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks Xuechuan Hong, Hak Soo Choi and Xiaoyuan Chen for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–31, Table 1 and Methods.
Supplementary Data 1
Source data for Supplementary Figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Shi, B., Gao, Y. et al. Ultrabright near-infrared fluorescent DNA frameworks for near-single-cell cancer imaging. Nat. Photon. 19, 79–88 (2025). https://doi.org/10.1038/s41566-024-01543-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41566-024-01543-7
This article is cited by
-
Fluorescence lifetime clocks quantify senescence and aging
Nature Aging (2025)
-
Single-shot X-ray and near-infrared (NIR) dual-mode fusion imaging based on bifunctional NIR scintillators
Light: Science & Applications (2025)