Abstract
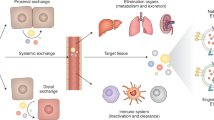
Communication between multicellular organs is essential to propagate signals and coordinate their function. Over the past decade, the role of extracellular vesicles in the molecular communication between cells in both physiological and pathological settings has received much attention. Extracellular vesicles can shuttle proteins, lipids and nucleic acids (such as RNA) between cells, thus inducing an array of functional changes in the recipient cells. In this Review, we describe the different extracellular vesicle subclasses and their heterogeneous nature, provide insights into extracellular vesicle-mediated signalling in the cardiovascular system, and highlight how extracellular vesicles can be used as diagnostic and prognostic biomarkers for a variety of pathological conditions. Finally, we also discuss the potential therapeutic applications of extracellular vesicles.
Key points
-
Extracellular vesicles (EVs) are effective mediators of cellular communication by transporting nucleic acids, proteins and lipids.
-
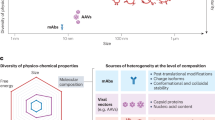
EVs are a heterogeneous population of vesicles that vary in size, cargo content, surface characteristics and intracellular origin, with differing pathways of biogenesis.
-
EVs or EV-linked molecules can be used to predict the progression and severity of numerous cardiovascular diseases.
-
Numerous techniques to isolate EVs have been employed for research, but they require careful validation for different applications given the differences in EV populations or profiles depending on the technique used.
-
A better understanding of the unique characteristics of EVs for intercellular communication will provide insights into how they can be used for diagnostic and therapeutic applications and will facilitate the design of better vehicles for drug delivery to reduce off-target effects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Townsend, N. et al. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 19, 133–143 (2022).
Perrino, C. et al. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 113, 725–736 (2017).
Sluijter, J. P. G. et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 114, 19–34 (2018).
Yates, A. G. et al. In sickness and in health: the functional role of extracellular vesicles in physiology and pathology in vivo. Part I: Health and normal physiology. J. Extracell. Vesicles 11, e12151 (2022).
Yates, A. G. et al. In sickness and in health: the functional role of extracellular vesicles in physiology and pathology in vivo. Part II: Pathology. J. Extracell. Vesicles 11, e12190 (2022).
Lässer, C., Jang, S. C. & Lötvall, J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol. Asp. Med. 60, 1–14 (2018).
Li, G. et al. Current challenges and future directions for engineering extracellular vesicles for heart, lung, blood and sleep diseases. J. Extracell. Vesicles 12, e12305 (2023).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles 13, e1240 (2024).
Davidson, S. M. et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles. Cardiovasc. Res. 119, 45–63 (2023).
Chen, T., Guo, J., Yang, M., Zhu, X. & Cao, X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 186, 2219–2228 (2011).
Kim, J.-S. et al. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. J. Physiol. Heart Circ. Physiol. 309, 425–433 (2015).
de Jong, O. G. et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 1, 18396 (2012).
Bari, E. et al. Silk fibroin bioink for 3D printing in tissue regeneration: controlled release of MSC extracellular vesicles. Pharmaceutics 15, 383 (2023).
Leroyer, A. S. et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation 119, 2808–2817 (2009).
Van De Wakker, S. I., Meijers, F. M., Sluijter, J. P. G. & Vader, P. Extracellular vesicle heterogeneity and its impact for regenerative medicine applications. Pharmacol. Rev. 75, 1043–1061 (2023).
van de Wakker, S. I. et al. Size matters: functional differences of small extracellular vesicle subpopulations in cardiac repair responses. J. Extracell. Vesicles 13, e12396 (2024).
Wang, X. et al. Essential role of Alix in regulating cardiomyocyte exosome biogenesis under physiological and stress conditions. J. Mol. Cell Cardiol. 190, 35–47 (2024).
Sun, R. et al. ALIX increases protein content and protective function of iPSC-derived exosomes. J. Mol. Med. 97, 829–844 (2019).
Oerlemans, M. I. F. J. et al. Targeting cell death in the reperfused heart: pharmacological approaches for cardioprotection. Int. J. Cardiol. 165, 410–422 (2013).
Gupta, K. et al. Necroptosis is associated with Rab27-independent expulsion of extracellular vesicles containing RIPK3 and MLKL. J. Extracell. Vesicles 11, e12261 (2022).
Maring, J. A. et al. Cardiac progenitor cell-derived extracellular vesicles reduce infarct size and associate with increased cardiovascular cell proliferation. J. Cardiovasc. Transl. Res. 12, 5–17 (2019).
Ortega, F. G. et al. Interfering with endolysosomal trafficking enhances release of bioactive exosomes. Nanomedicine 20, 102014 (2019).
Fordjour, F. K. et al. Exomap1 mouse: a transgenic model for in vivo studies of exosome biology. Extracell. Vesicles 2, 100030 (2023).
Van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Van Deun, J. et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 14, 228–232 (2017).
Fu, S. et al. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 6, 68 (2020).
Cheung, S. W. Y., Chamley, L. W., Barrett, C. J. & Lau, S. Y. S. Extracellular vesicles and their effect on vascular haemodynamics: a systematic review. Hypertens. Res. 10, e12085 (2024).
Pironti, G. et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131, 2120–2130 (2015).
Good, M. E. et al. Circulating extracellular vesicles in normotension restrain vasodilation in resistance arteries. Hypertension 75, 218–228 (2020).
Webber, R. J., Sweet, R. M. & Webber, D. S. Inducible nitric oxide synthase in circulating microvesicles: discovery, evolution, and evidence as a novel biomarker and the probable causative agent for sepsis. J. Appl. Laboratory Med. 3, 698–711 (2019).
Lee, Y. J. et al. Circulating small extracellular vesicles promote proliferation and migration of vascular smooth muscle cells via AXL and MerTK activation. Acta Pharmacol. Sin. 44, 984–998 (2023).
Royo, F. et al. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci. Rep. 7, 42798 (2017).
Raeven, P., Zipperle, J. & Drechsler, S. Extracellular vesicles as markers and mediators in sepsis. Theranostics 8, 3348–3365 (2018).
Wei, G. et al. Extracellular vesicle-derived CircWhsc1 promotes cardiomyocyte proliferation and heart repair by activating TRIM59/STAT3/cyclin B2 pathway. J. Adv. Res. 53, 199–218 (2023).
Hegyesi, H. et al. Circulating cardiomyocyte-derived extracellular vesicles reflect cardiac injury during systemic inflammatory response syndrome in mice. Cell. Mol. Life Sci. 79, 84 (2022).
Rodriguez, J. A. et al. Selective increase of cardiomyocyte derived extracellular vesicles after experimental myocardial infarction and functional effects on the endothelium. Thromb. Res. 170, 1–9 (2018).
Almeida Paiva, R. et al. Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J. Cell Mol. Med. 23, 1137–1151 (2019).
Zhou, Y. et al. Peptide-anchored biomimetic interface for electrochemical detection of cardiomyocyte-derived extracellular vesicles. Anal. Bioanal. Chem. 415, 1305–1311 (2023).
Terriaca, S. et al. Endothelial progenitor cell-derived extracellular vesicles: potential therapeutic application in tissue repair and regeneration. Int. J. Mol. Sci. 22, 6375 (2021).
Li, L. et al. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 7, 235 (2021).
Chen, C. W. et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 114, 1029–1040 (2018).
Zheng, S. et al. microRNA-129 overexpression in endothelial cell-derived extracellular vesicle influences inflammatory response caused by myocardial ischemia/reperfusion injury. Cell Biol. Int. 8, 1743–1756 (2021).
Yadid, M. et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci. Transl. Med. 12, eaax8005 (2020).
Peters, M. M. C., Sampaio-Pinto, V. & da Costa Martins, P. A. Non-coding RNAs in endothelial cell signalling and hypoxia during cardiac regeneration. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118515 (2020).
Zhu, Y., Liao, Z. F., Mo, M. H. & Xiong, X. D. Mesenchymal stromal cell-derived extracellular vesicles for vasculopathies and angiogenesis: therapeutic applications and optimization. Biomolecules 13, 1109 (2023).
Spinosa, M. et al. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. 32, fj201701138RR (2018).
Zhang, C., Wang, H., Li, J. & Ma, L. Circular RNA involvement in the protective effect of human umbilical cord mesenchymal stromal cell-derived extracellular vesicles against hypoxia/reoxygenation injury in cardiac cells. Front. Cardiovasc. Med. 8, 626878 (2021).
Łabędź-Masłowska, A. et al. Mesenchymal stem cell-derived extracellular vesicles exert pro-angiogenic and pro-lymphangiogenic effects in ischemic tissues by transferring various microRNAs and proteins including ITGa5 and NRP1. J. Nanobiotechnol. 22, 60 (2024).
Malvicini, R. et al. Influence of the isolation method on characteristics and functional activity of mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 26, 157–170 (2024).
Hsu, C. Y. et al. Characterization and proteomic analysis of endometrial stromal cell-derived small extracellular vesicles. J. Clin. Endocrinol. Metab. 106, 1516–1529 (2021).
Liu, Q. et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Invest. 126, 2805–2820 (2016).
Yang, J. et al. T-cell-derived extracellular vesicles regulate B-cell IgG production via pyruvate kinase muscle isozyme 2. FASEB J. 33, 12780–12799 (2019).
Lugo-Gavidia, L. M. et al. Circulating platelet-derived extracellular vesicles correlate with night-time blood pressure and vascular organ damage and may represent an integrative biomarker of vascular health. J. Clin. Hypertens. 24, 738–749 (2022).
Kong, L. et al. Mediating effects of platelet-derived extracellular vesicles on PM2.5-induced vascular endothelial injury. Ecotoxicol. Env. Saf. 198, 110652 (2020).
Manakeng, K. et al. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent β-thalassemia/HbE patients with pulmonary arterial hypertension. Ann. Hematol. 98, 281–288 (2019).
Reddel, C. J., Pennings, G. J., Lau, J. K., Chen, V. M. & Kritharides, L. Circulating platelet-derived extracellular vesicles are decreased after remote ischemic preconditioning in patients with coronary disease: a randomized controlled trial. J. Thrombosis Haemost. 19, 2605–2611 (2021).
Lugo-Gavidia, L. M. et al. Platelet-derived extracellular vesicles correlate with therapy-induced nocturnal blood pressure changes. J. Hypertens. 40, 2210–2218 (2022).
Zhu, Z. et al. A double-edged sword of platelet-derived extracellular vesicles in tissues, injury or repair: the current research overview. Tissue Cell 82, 102066 (2023).
Al Halawani, A., Mithieux, S. M., Yeo, G. C., Hosseini-Beheshti, E. & Weiss, A. S. Extracellular vesicles: interplay with the extracellular matrix and modulated cell responses. Int. J. Mol. Sci. 23, 3389 (2022).
Dolo, V. et al. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin. Exp. Metastasis 17, 131–140 (1999).
Ruiz, J. L., Weinbaum, S., Aikawa, E. & Hutcheson, J. D. Zooming in on the genesis of atherosclerotic plaque microcalcifications. J. Physiol. 594, 2915–2927 (2016).
Rogers, M. A. et al. Annexin A1-dependent tethering promotes extracellular vesicle aggregation revealed with single-extracellular vesicle analysis. Sci. Adv. 6, eabb1244 (2020).
Kalra, H. et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 10, e1001450 (2012).
Blaser, M. C. et al. Multiomics of tissue extracellular vesicles identifies unique modulators of atherosclerosis and calcific aortic valve stenosis. Circulation 148, 661–678 (2023).
Das, S., Lyon, C. J. & Hu, T. A panorama of extracellular vesicle applications: from biomarker detection to therapeutics. ACS Nano 18, 9784–9797 (2024).
Tian, C., Gao, L., Rudebush, T. L., Yu, L. & Zucker, I. H. Extracellular vesicles regulate sympatho-excitation by Nrf2 in heart failure. Circ. Res. 131, 687–700 (2022).
Matan, D. et al. Extracellular vesicles in heart failure – a study in patients with heart failure with preserved ejection fraction or heart failure with reduced ejection fraction characteristics undergoing elective coronary artery bypass grafting. Front. Cardiovasc. Med. 9, 952974 (2022).
Wang, L., Liu, J., Xu, B., Liu, Y. L. & Liu, Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J. Med. Sci. 34, 626–633 (2018).
Wu, T. et al. Circulating exosomal miR-92b-5p is a promising diagnostic biomarker of heart failure with reduced ejection fraction patients hospitalized for acute heart failure. J. Thorac. Dis. 10, 6211–6220 (2018).
Matsumoto, S. et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 113, 322–326 (2013).
Khandagale, A. et al. MircoRNA in extracellular vesicles from patients with pulmonary arterial hypertension alters endothelial angiogenic response. Int. J. Mol. Sci. 23, 11964 (2022).
Khandagale, A. et al. Role of extracellular vesicles in pulmonary arterial hypertension modulation of pulmonary endothelial function and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 40, 2293–2309 (2020).
de Hoog, V. C. et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2, 53–60 (2013).
Steogonekpień, E. et al. Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch. Med. Res. 43, 31–35 (2012).
Oerlemans, M. I. F. J. et al. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol. Med. 4, 1176–1185 (2012).
Mørk, M. et al. Elevated blood plasma levels of tissue factor-bearing extracellular vesicles in patients with atrial fibrillation. Thromb. Res. 173, 141–150 (2019).
Bai, C. et al. Circulating exosome-derived miR-122-5p is a novel biomarker for prediction of postoperative atrial fibrillation. J. Cardiovasc. Transl. Res. 15, 1393–1405 (2022).
Kang, J. Y., Mun, D., Kim, H., Yun, N. & Joung, B. Serum exosomal long noncoding RNAs as a diagnostic biomarker for atrial fibrillation. Heart Rhythm. 19, 1450–1458 (2022).
Zhang, Y. et al. Characterization of circRNA-associated ceRNA networks in patients with nonvalvular persistent atrial fibrillation. Mol. Med. Rep. 19, 638–650 (2019).
Joo, S. J. et al. Transcriptomic landscape of circulating extracellular vesicles in heart transplant ischemia–reperfusion. Genes 14, 2101 (2023).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211 (2017).
Arslan, F. et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301–312 (2013).
Correa, B. L. et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc. Res. 117, 292–307 (2021).
Roefs, M. T. et al. Cardiac progenitor cell-derived extracellular vesicles promote angiogenesis through both associated- and co-isolated proteins. Commun. Biol. 6, 800 (2023).
Rogers, R. G., Ciullo, A., Marbán, E. & Ibrahim, A. G. Extracellular vesicles as therapeutic agents for cardiac fibrosis. Front. Physiol. 11, 479 (2020).
Cheng, G., Zhu, D., Huang, K. & Caranasos, T. G. Minimally invasive delivery of a hydrogel-based exosome patch to prevent heart failure. J. Mol. Cell Cardiol. 169, 113–121 (2022).
Deddens, J. C. et al. Circulating extracellular vesicles contain miRNAs and are released as early biomarkers for cardiac injury. J. Cardiovasc. Transl. Res. 9, 291–301 (2016).
Vicencio, J. M. et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 65, 1525–1536 (2015).
Deddens, J. C., Vrijsen, K. R., Girao, H., Doevendans, P. A. & Sluijter, J. P. G. Cardiac-released extracellular vesicles can activate endothelial cells. Ann. Transl. Med. 5, 64 (2017).
Giricz, Z. et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J. Mol. Cell Cardiol. 68, 75–78 (2014).
Wang, H. et al. Percutaneous intracoronary delivery of plasma extracellular vesicles protects the myocardium against ischemia-reperfusion injury in canis. Hypertension 78, 1541–1554 (2021).
Gollmann-Tepekoylu, C. et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc. Res. 116, 1226–1236 (2021).
Sun, P. et al. Low-intensity pulsed ultrasound protects from inflammatory dilated cardiomyopathy through inciting extracellular vesicles. Cardiovasc. Res. 120, 1177–1190 (2024).
Kang, J. Y. et al. Engineered small extracellular vesicle-mediated NOX4 siRNA delivery for targeted therapy of cardiac hypertrophy. J. Extracell. Vesicles 12, e12371 (2023).
de Boer, C. & Davies, N. H. Blood derived extracellular vesicles as regenerative medicine therapeutics. Biochimie 196, 203–215 (2022).
Livkisa, D. et al. Extracellular vesicles purified from serum-converted human platelet lysates offer strong protection after cardiac ischaemia/reperfusion injury. Biomaterials 306, 122502 (2024).
Soler-Botija, C. et al. Mechanisms governing the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles: a scoping review of preclinical evidence. Biomed. Pharmacother. 147, 112683 (2022).
Roefs, M. T., Sluijter, J. P. G. & Vader, P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 30, 990–1013 (2020).
Barile, L. et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc. Res. 114, 992–1005 (2018).
Emmert, M. et al. Intracoronary delivery of extracellular vesicles from human cardiac progenitor cells reduces infarct size in porcine acute myocardial infarction. Eur. Heart J. 45, 728–732 (2024).
Xing, Z., Zhao, C., Liu, H. & Fan, Y. Endothelial progenitor cell-derived extracellular vesicles: a novel candidate for regenerative medicine and disease treatment. Adv. Healthc. Mater. 9, e2000255 (2020).
Yu, B. et al. Extracellular vesicles engineering by silicates-activated endothelial progenitor cells for myocardial infarction treatment in male mice. Nat. Commun. 14, 2094 (2023).
Khan, K. et al. Extracellular vesicles as a cell-free therapy for cardiac repair: a systematic review and meta-analysis of randomized controlled preclinical trials in animal myocardial infarction models. Stem Cell Rev. Rep. 18, 1143–1167 (2022).
Yang, L. et al. Stem cell-derived extracellular vesicles for myocardial infarction: a meta-analysis of controlled animal studies. Aging 11, 1129–1150 (2019).
Zwetsloot, P. P. et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ. Res. 118, 1223–1232 (2016).
De Jong, O. G. et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc. Chem. Res. 52, 1761–1770 (2019).
Kooijmans, S. A. A. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Controlled Rel. 172, 229–238 (2013).
Fabiani, M. et al. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Eurosurveillance 26, 2100420 (2021).
Urits, I. et al. A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol. Ther. 9, 301–315 (2020).
Murphy, D. E. et al. Natural or synthetic RNA delivery: a stoichiometric comparison of extracellular vesicles and synthetic nanoparticles. Nano Lett. 21, 1888–1895 (2021).
Lee, Y., Jeong, M., Park, J., Jung, H. & Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 55, 2085–2096 (2023).
Evers, M. J. W. et al. Functional siRNA delivery by extracellular vesicle–liposome hybrid nanoparticles. Adv. Healthc. Mater. 11, e2101202 (2022).
Ilahibaks, N. F. et al. TOP-EVs: technology of protein delivery through extracellular vesicles is a versatile platform for intracellular protein delivery. J. Controlled Rel. 355, 579–592 (2023).
Ilahibaks, N. F. et al. Extracellular vesicle-mediated delivery of CRISPR/Cas9 ribonucleoprotein complex targeting proprotein convertase subtilisin-kexin type 9 (Pcsk9) in primary mouse hepatocytes. J. Extracell. Vesicles 13, e12389 (2024).
Ivanova, A. et al. Creating designer engineered extracellular vesicles for diverse ligand display, target recognition, and controlled protein loading and delivery. Adv. Sci. 10, e2304389 (2023).
Van der Spoel, T. I. G. et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: a study on delivery efficiency. J. Cell Mol. Med. 16, 2768–2776 (2012).
van den Akker, F. et al. Intramyocardial stem cell injection: go(ne) with the flow. Eur. Heart J. 38, 184–186 (2017).
Roefs, M. T. et al. Evaluation and manipulation of tissue and cellular distribution of cardiac progenitor cell-derived extracellular vesicles. Front. Pharmacol. 13, 1052091 (2022).
Wiklander, O. P. B. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 (2015).
Lázaro-Ibánez, E. et al. Selection of fluorescent, bioluminescent, and radioactive tracers to accurately reflect extracellular vesicle biodistribution in vivo. ACS Nano 15, 3212–3227 (2021).
Xu, C. M. et al. Visualization of cardiac uptake of bone marrow mesenchymal stem cell-derived extracellular vesicles after intramyocardial or intravenous injection in murine myocardial infarction. Physiol. Rep. 11, e15568 (2023).
Mol, E. A. et al. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv. Healthc. Mater. 8, 1900847 (2019).
Monguió-Tortajada, M. et al. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics 6, 4656–4670 (2022).
Gao, H. et al. Injectable hydrogel-based combination therapy for myocardial infarction: a systematic review and meta-analysis of preclinical trials. BMC Cardiovasc. Disord. 24, 119 (2024).
Zou, Y. et al. Restoring cardiac functions after myocardial infarction-ischemia/reperfusion via an exosome anchoring conductive hydrogel. ACS Appl. Mater. Interfaces 13, 56892–56908 (2021).
Zhu, D. et al. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 12, 1412 (2021).
Yuan, J. et al. Microneedle patch loaded with exosomes containing microRNA-29b prevents cardiac fibrosis after myocardial infarction. Adv. Healthc. Mater. 12, e2202959 (2023).
Gu, J. et al. Engineered bone marrow mesenchymal stem cell-derived exosomes loaded with miR302 through the cardiomyocyte specific peptide can reduce myocardial ischemia and reperfusion (I/R) injury. J. Transl. Med. 22, 168 (2024).
Mun, D., Kang, J.-Y., Kim, H., Yun, N. & Joung, B. Small extracellular vesicle-mediated CRISPR-Cas9 RNP delivery for cardiac-specific genome editing. J. Controlled Rel. 370, 798–810 (2024).
Tian, Y. et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles 9, 1697028 (2020).
Guo, J. et al. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J. Extracell. Vesicles 10, e12145 (2021).
Escudé Martinez de Castilla, P. et al. Extracellular vesicles as a drug delivery system: a systematic review of preclinical studies. Adv. Drug. Delivery Rev. 175, 113801 (2021).
Courageux, Y. et al. Clinical translation of mesenchymal stromal cell extracellular vesicles: considerations on scientific rationale and production requisites. J. Cell Mol. Med. 26, 937–939 (2022).
Saftics, A. et al. Single extracellular vesicle nanoscopy. J. Extracell. Vesicles 12, e12346 (2023).
Bettin, B. A., Varga, Z., Nieuwland, R. & van der Pol, E. Standardization of extracellular vesicle concentration measurements by flow cytometry: the past, present, and future. J. Thrombosis Haemost. 21, 2032–2044 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT03674255 (2020).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06298682 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05524506 (2022).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05584943 (2022).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06169540 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04731987 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06071559 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06209359 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05323487 (2025).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT03249532 (2021).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06319742 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06275399 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06408961 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06316271 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05199454 (2025).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT02931045 (2020).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04698447 (2021).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05774509 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05243368 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04652531 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06319287 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06198543 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04578223 (2020).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05855317 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04191044 (2019).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05490173 (2022).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05645081 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05326724 (2022).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04266639 (2022).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06257823 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT02458755 (2017).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT03481777 (2025).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05370105 (20252).
Buntsma, N. C. et al. EDTA stabilizes the concentration of platelet-derived extracellular vesicles during blood collection and handling. Platelets 33, 764–771 (2022).
International Society for Extracellular Vesicles. Scientific Reproducibility Task Forces. ISEV www.isev.org/taskforces (2021).
Johnsen, K. B., Gudbergsson, J. M., Andresen, T. L. & Simonsen, J. B. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim. Biophys. Acta Rev. Cancer 1871, 109–116 (2019).
Wolf, M. et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell. Vesicles 11, e12207 (2022).
Lucien, F. et al. MIBlood-EV: minimal information to enhance the quality and reproducibility of blood extracellular vesicle research. J. Extracell. Vesicles 12, e12385 (2023).
Lehrich, B. M., Liang, Y. & Fiandaca, M. S. Foetal bovine serum influence on in vitro extracellular vesicle analyses. J. Extracell. Vesicles 10, e12061 (2021).
Lehrich, B. M., Liang, Y., Khosravi, P., Federoff, H. J. & Fiandaca, M. S. Fetal bovine serum-derived extracellular vesicles persist within vesicle-depleted culture media. Int. J. Mol. Sci. 19, 3538 (2018).
Shlomovitz, I. et al. Proteomic analysis of necroptotic extracellular vesicles. Cell Death Dis. 12, 1059 (2021).
Hurwitz, S. N., Olcese, J. M. & Meckes, D. G. Extraction of extracellular vesicles from whole tissue. J. Vis. Exp. https://doi.org/10.3791/59143 (2019).
Acknowledgements
The authors’ work was supported by grants from the National Natural Science Foundation of China (82225005 and 82020108002 to J.X.) and the Science and Technology Commission of Shanghai Municipality (23410750100, 20DZ2255400 and 21XD1421300 to J.X.). J.P.G.S. is supported by H2020-EVICARE (#725229) and TOP-EVICARE (#101138069) of the European Research Council, and by ZonMw Psider-Heart (10250022110004) and NWO-TTP HARVEY (2021/TTW/01038252), Health-Holland 2022TKI2306 EV-PROTECT and ERA for Health Cardinnov (RESCUE- 2024/KIC/01627794).
Author information
Authors and Affiliations
Contributions
Both authors contributed to researching data for the article, discussion of content, writing the article, and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Lucio Barile, Santiago Roura and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Sluijter, J.P.G. Extracellular vesicles in cardiovascular homeostasis and disease: potential role in diagnosis and therapy. Nat Rev Cardiol 22, 883–895 (2025). https://doi.org/10.1038/s41569-025-01141-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-025-01141-2
This article is cited by
-
Exosomes: bridge metabolic regulation in cardiac repair
npj Biomedical Innovations (2025)
-
Extracellular Vesicles in Cardiac Cell Crosstalk: from Intercellular Communication to Clinical Translation
Cardiovascular Drugs and Therapy (2025)