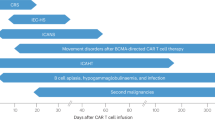
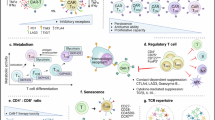
Abstract
Cancer immunotherapies are associated with remarkable therapeutic response rates but also with unique and severe toxicities, which potentially result in rapid deterioration in health. The number of clinical applications for novel immune effector-cell therapies, including chimeric antigen receptor (CAR)-expressing cells, and other immunotherapies, such as immune-checkpoint inhibitors, is increasing. In this Consensus Statement, members of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Hematopoietic Cell Transplantation-Cancer Immunotherapy (HCT-CI) Subgroup, Paediatric Diseases Working Party (PDWP) of the European Society of Blood and Marrow Transplantation (EBMT), Supportive Care Committee of the Pediatric Transplantation and Cellular Therapy Consortium (PTCTC) and MD Anderson Cancer Center CAR T Cell Therapy-Associated Toxicity (CARTOX) Program collaborated to provide updated comprehensive recommendations for the care of children, adolescents and young adults receiving cancer immunotherapies. With these recommendations, we address emerging toxicity mitigation strategies, we advocate for the characterization of baseline organ function according to age and discipline-specific criteria, we recommend early critical care assessment when indicated, with consideration of reversibility of underlying pathology (instead of organ failure scores) to guide critical care interventions, and we call for researchers, regulatory agencies and sponsors to support and facilitate early inclusion of young patients with cancer in well-designed clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
17 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41571-021-00497-x
References
Palmer, S., Patterson, P. & Thompson, K. A national approach to improving adolescent and young adult (AYA) oncology psychosocial care: the development of AYA-specific psychosocial assessment and care tools. Palliat. Support. Care 12, 183–188 (2014).
Mahadeo, K. M. et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat. Rev. Clin. Oncol. 16, 45–63 (2019).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019).
FDA. Highlights of Prescribing Information: KYMRIAH. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert---KYMRIAH.pdf (2018).
FDA. Highlights of Prescribing Information: YESCARTA. https://www.fda.gov/media/108377/download (2020).
FDA. Highlights of Prescribing Information: TECARTUS. https://www.fda.gov/media/140409/download (2020).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Li, D. et al. Genetically engineered T cells for cancer immunotherapy. Signal. Transduct. Target. Ther. 4, 35 (2019).
Johnson, L. A. & June, C. H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 27, 38–58 (2017).
Baiden-Amissah, R. E. M. & Tuyaerts, S. Contribution of aging, obesity, and microbiota on tumor immunotherapy efficacy and toxicity. Int. J. Mol. Sci. 20, 3586 (2019).
Ruella, M. & Maus, M. V. Catch me if you can: leukemia escape after CD19-directed T cell immunotherapies. Comput. Struct. Biotechnol. J. 14, 357–362 (2016).
Fousek, K. et al. CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression. Leukemia 35, 75–89 (2021).
György, B. Bispecific CAR T cells have a dual grasp on tumors. Sci. Transl. Med. 12, eabf2636 (2020).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Ghorashian, S. et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 25, 1408–1414 (2019).
Benjamin, R. et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 (2020).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Scott, M. C. et al. Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res. 78, 326–337 (2018).
Serrels, A. et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell 163, 160–173 (2015).
Rosenberg, S. A. et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N. Engl. J. Med. 319, 1676–1680 (1988).
Lizee, G. et al. Harnessing the power of the immune system to target cancer. Annu. Rev. Med. 64, 71–90 (2013).
Gattinoni, L., Powell, D. J. Jr., Rosenberg, S. A. & Restifo, N. P. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol. 6, 383–393 (2006).
Wang, Z., Li, B., Ren, Y. & Ye, Z. T-cell-based immunotherapy for osteosarcoma: challenges and opportunities. Front. Immunol. 7, 353 (2016).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Rosenberg, S. A. et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl Cancer Inst. 86, 1159–1166 (1994).
Parkhurst, M. R. et al. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 9, 1022–1035 (2019).
Turajlic, S. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021 (2017).
Olle Hurtado, M. et al. Tumor infiltrating lymphocytes expanded from pediatric neuroblastoma display heterogeneity of phenotype and function. PLoS ONE 14, e0216373 (2019).
Mullinax, J. E. et al. Combination of ipilimumab and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma. Front. Oncol. 8, 44 (2018).
Zhao, Q. et al. Tumor-targeted IL-12 combined with tumor resection yields a survival-favorable immune profile. J. Immunother. Cancer 7, 154 (2019).
Nguyen, L. T. et al. Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2. Cancer Immunol. Immunother. 68, 773–785 (2019).
Schwinger, W. et al. Feasibility of high-dose interleukin-2 in heavily pretreated pediatric cancer patients. Ann. Oncol. 16, 1199–1206 (2005).
Yeh, S. et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology 116, 981–989.e1 (2009).
Robbins, P. F. et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21, 1019–1027 (2015).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Chodon, T. et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 20, 2457–2465 (2014).
Tawara, I. et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 130, 1985–1994 (2017).
Rezvani, K., Rouce, R., Liu, E. & Shpall, E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 25, 1769–1781 (2017).
Khatua, S. et al. Phase I study of intraventricular infusions of autologous ex-vivo-expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncol. 22, 1214–1225 (2020).
Screpanti, V., Wallin, R. P., Ljunggren, H. G. & Grandien, A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J. Immunol. 167, 2068–2073 (2001).
Vela, M. et al. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett. 422, 107–117 (2018).
Rubnitz, J. E. et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 28, 955–959 (2010).
Nguyen, R. et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J. Immunother. Cancer 7, 81 (2019).
Cho, D. et al. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin. Cancer Res. 16, 3901–3909 (2010).
Fernandez, L. et al. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. 368, 54–63 (2015).
Shah, N. N. et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 125, 784–792 (2015).
Pérez-Martínez, A. et al. Natural killer cells can exert a graft-vs-tumor effect in haploidentical stem cell transplantation for pediatric solid tumors. Exp. Hematol. 40, 882–891.e1 (2012).
Kimpo, M. S., Oh, B. & Lee, S. The role of natural killer cells as a platform for immunotherapy in pediatric cancers. Curr. Oncol. Rep. 21, 93 (2019).
Denman, C. J. et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 7, e30264 (2012).
Andersen, M. H., Schrama, D., thor Straten, P. & Becker, J. C. Cytotoxic T cells. J. Invest. Dermatol. 126, 32–41 (2006).
Van Pel, A. & Boon, T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc. Natl Acad. Sci. USA 79, 4718–4722 (1982).
Castelli, C. et al. T-cell recognition of melanoma-associated antigens. J. Cell. Physiol. 182, 323–331 (2000).
Liu, Z. et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent. Results Cancer Res. 159, 123–133 (2002).
Prockop, S. et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J. Clin. Invest. 130, 733–747 (2020).
Moosmann, A. et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 115, 2960–2970 (2010).
Prockop, S. E. et al. A multicenter, open label, phase 3 study of tabelecleucel for solid organ transplant subjects with Epstein-Barr virus-driven post-transplant lymphoproliferative disorder (EBV+PTLD) after failure of rituximab or rituximab and chemotherapy (ALLELE) [abstract]. Biol. Blood Marrow Transpl. 26, S274 (2020).
Prockop, S. E. et al. Long-term outcomes of patients with epstein-barr virus-driven post-transplant lymphoproliferative disease following solid organ transplant or allogeneic hematopoietic cell transplant treated with tabelecleucel in a multicenter expanded access program study [abstract]. Biol. Blood Marrow Transpl. 26, S61–S62 (2020).
Bollard, C. M. et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+Hodgkin’s disease. J. Exp. Med. 200, 1623–1633 (2004).
Straathof, K. C. et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood 105, 1898–1904 (2005).
Schuessler, A. et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 74, 3466–3476 (2014).
Leen, A. M. et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013).
Gottschalk, S. & Rooney, C. M. Adoptive T-cell immunotherapy. Curr. Top. Microbiol. Immunol. 391, 427–454 (2015).
Muftuoglu, M. et al. Allogeneic BK virus-specific T cells for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 379, 1443–1451 (2018).
Pello, O. M. et al. BKV-specific T cells in the treatment of severe refractory haemorrhagic cystitis after HLA-haploidentical haematopoietic cell transplantation. Eur. J. Haematol. 98, 632–634 (2017).
Huehls, A. M., Coupet, T. A. & Sentman, C. L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 93, 290–296 (2015).
FDA. Highlights of Prescribing Information: Blincyto. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125557s013lbl.pdf (2018).
Boussiotis, V. A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 375, 1767–1778 (2016).
Toor, S. M. & Elkord, E. Therapeutic prospects of targeting myeloid-derived suppressor cells and immune checkpoints in cancer. Immunol. Cell Biol. 96, 888–897 (2018).
Hargadon, K. M., Johnson, C. E. & Williams, C. J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 62, 29–39 (2018).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Merchant, M. S. et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin. Cancer Res. 22, 1364–1370 (2016).
Geoerger, B. et al. Phase II study of ipilimumab in adolescents with unresectable stage III or IV malignant melanoma. Eur. J. Cancer 86, 358–363 (2017).
Davis, K. L. et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 21, 541–550 (2020).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Overman, M. J. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36, 773–779 (2018).
Geoerger, B. et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 21, 121–133 (2020).
Goldberg, S. B. et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 21, 655–663 (2020).
Kawazoe, A. et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 21, 1057–1065 (2020).
Grosser, R., Cherkassky, L., Chintala, N. & Adusumilli, P. S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36, 471–482 (2019).
Ghosh, A. K. et al. CAR T cell therapy-related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? JACC 2, 97–109 (2020).
Feng, Y. et al. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin. Cancer Res. 19, 3977–3986 (2013).
Ihara, K. Immune checkpoint inhibitor therapy for pediatric cancers: a mini review of endocrine adverse events. Clin. Pediatr. Endocrinol. 28, 59–68 (2019).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Brahmer, J. R. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 36, 1714–1768 (2018).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Queudeville, M. et al. Blinatumomab in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Eur. J. Haematol. https://doi.org/10.1111/ejh.13569 (2020).
Berg, M. D. et al. Part 13: Pediatric basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122, S862–S875 (2010).
Gutierrez, C. et al. Critical care management of toxicities associated with targeted agents and immunotherapies for cancer. Crit. Care Med. 48, 10–21 (2020).
Fitzgerald, J. C. et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 45, e124–e131 (2017).
Roy, J. P. & Devarajan, P. Acute kidney injury: diagnosis and management. Indian J. Pediatr. 87, 600–607 (2020).
Fragasso, T., Ricci, Z. & Goldstein, S. L. in Contributions to Nephrology (eds Ding, X., Rosner, M. H. & Ronco, C.) 113–126 (Karger, 2018). [Series Ed. Ronco, C. Acute Kidney Injury - Basic Research and Clinical Practice Vol. 193]
Menon, S. et al. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol. Dial. Transpl. 31, 586–594 (2016).
Bottari, G. et al. Multimodal therapeutic approach of cytokine release syndrome developing in a child given chimeric antigen receptor-modified T cell infusion. Crit. Care Explor. 2, e0071 (2020).
Gutgarts, V. et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol. Blood Marrow Transpl. 26, 1071–1076 (2020).
Gupta, S. et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am. J. Kidney Dis. 76, 63–71 (2020).
Gutierrez, C. et al. The chimeric antigen receptor–intensive care unit (CAR-ICU) initiative: surveying intensive care unit practices in the management of CAR T-cell associated toxicities. J. Crit. Care 58, 58–64 (2020).
Dubois, M. J. et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: a prospective, randomized, controlled, pilot study. Crit. Care Med. 34, 2536–2540 (2006).
Hariri, G. et al. Albumin infusion improves endothelial function in septic shock patients: a pilot study. Intensive Care Med. 44, 669–671 (2018).
Hu, Y., Feng, J., Shao, M. & Huang, H. Profile of capillary-leak syndrome in patients received chimeric antigen receptor T cell therapy [abstract]. Blood 132 (Suppl. 1), 5204 (2018).
Milesi, C. et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann. Intensive Care 4, 29 (2014).
Dysart, K., Miller, T. L., Wolfson, M. R. & Shaffer, T. H. Research in high flow therapy: mechanisms of action. Respir. Med. 103, 1400–1405 (2009).
McDonald, C. F. Low-flow oxygen: how much is your patient really getting? Respirology 19, 469–470 (2014).
Kwon, J. W. High-flow nasal cannula oxygen therapy in children: a clinical review. Clin. Exp. Pediatr. 63, 3–7 (2020).
Hutchings, F. A., Hilliard, T. N. & Davis, P. J. Heated humidified high-flow nasal cannula therapy in children. Arch. Dis. Child. 100, 571–575 (2015).
Weiler, T. et al. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J. Pediatr. 189, 66–71.e3 (2017).
Morley, S. L. Non-invasive ventilation in paediatric critical care. Paediatr. Respir. Rev. 20, 24–31 (2016).
Morris, J. V., Kapetanstrataki, M., Parslow, R. C., Davis, P. J. & Ramnarayan, P. Patterns of use of heated humidified high-flow nasal cannula therapy in PICUs in the United Kingdom and Republic of Ireland. Pediatr. Crit. Care Med. 20, 223–232 (2019).
Mestermann, K. et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 11, eaau5907 (2019).
Philip, B. RQR8: A universal safety switch for cellular therapies. Thesis, University College London (2015).
Gardner, R. A. et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 134, 2149–2158 (2019).
Kadauke, S. et al. Early administration of tocilizumab (Toci) for the prevention of grade 4 cytokine release syndrome (CRS) after CD19-directed CAR T-cell therapy (CTL019) [abstract]. Cytotherapy 21, e2–e3 (2019).
Yanez, L., Sanchez-Escamilla, M. & Perales, M. A. CAR T cell toxicity: current management and future directions. Hemasphere 3, e186 (2019).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Mahadeo, K. M. et al. Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome: an international expert position statement. Lancet Haematol. 7, e61–e72 (2020).
Gust, J. et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann. Neurol. 86, 42–54 (2019).
Gust, J. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Miettunen, P. M., Narendran, A., Jayanthan, A., Behrens, E. M. & Cron, R. Q. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology 50, 417–419 (2011).
Henter, J. I. et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 48, 124–131 (2007).
Brudno, J. N. et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 34, 1112–1121 (2016).
Glucksberg, H. et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18, 295–304 (1974).
Jacobsohn, D. Acute graft-versus-host disease in children. Bone Marrow Transplant. 41, 215–221 (2008).
Harris, A. C. et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transpl. 22, 4–10 (2016).
Li, X., Shao, C., Shi, Y. & Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 11, 31 (2018).
Liu, Y. H. et al. Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. Biomed. Pharmacother. 120, 109437 (2019).
Khan, M. et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine 97, e11936 (2018).
de La Rochefoucauld, J., Noel, N. & Lambotte, O. Management of immune-related adverse events associated with immune checkpoint inhibitors in cancer patients: a patient-centred approach. Intern. Emerg. Med. 15, 587–598 (2020).
Weber, J. S., Kahler, K. C. & Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697 (2012).
Li, A. M. et al. Checkpoint inhibitors augment CD19-directed chimeric antigen receptor (CAR) T cell therapy in relapsed B-cell acute lymphoblastic leukemia [abstract]. Blood 132 (Suppl. 1), 556 (2018).
Bajciova, V. Therapeutic effect and tolerance of ipilimumam in metastatic malignant melanoma in children – a case report. Klin. Onkol. 28, 4S115–4S120 (2015).
Blumenthal, D. T. et al. Pembrolizumab: first experience with recurrent primary central nervous system (CNS) tumors. J. Neurooncol 129, 453–460 (2016).
Bouffet, E. et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 34, 2206–2211 (2016).
Foran, A. E., Nadel, H. R., Lee, A. F., Savage, K. J. & Deyell, R. J. Nivolumab in the treatment of refractory pediatric Hodgkin lymphoma. J. Pediatr. Hematol. Oncol. 39, e263–e266 (2017).
Shad, A. T. et al. Tolerance and effectiveness of nivolumab after pediatric T-cell replete, haploidentical, bone marrow transplantation: a case report. Pediatr. Blood Cancer 64, e26257 (2017).
Zhu, X. et al. Severe cerebral edema following nivolumab treatment for pediatric glioblastoma: case report. J. Neurosurg. Pediatr. 19, 249–253 (2017).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Weber, J. S., Yang, J. C., Atkins, M. B. & Disis, M. L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 33, 2092–2099 (2015).
Trinh, S., Le, A., Gowani, S. & La-Beck, N. M. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac. J. Oncol. Nurs. 6, 154–160 (2019).
Livingston, E. H. & Lee, S. Percentage of burned body surface area determination in obese and nonobese patients. J. Surg. Res. 91, 106–110 (2000).
Ham, P. & Allen, C. Adolescent health screening and counseling. Am. Fam. Phys. 86, 1109–1116 (2012).
Clotman, K., Janssens, K., Specenier, P., Weets, I. & De Block, C. E. M. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 103, 3144–3154 (2018).
Stamatouli, A. M. et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 67, 1471–1480 (2018).
Sakamoto, K., Fukihara, J., Morise, M. & Hashimoto, N. Clinical burden of immune checkpoint inhibitor-induced pneumonitis. Respir. Investig. 58, 305–319 (2020).
Naidoo, J. et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J. Clin. Oncol. 35, 709–717 (2017).
Rashdan, S., Minna, J. D. & Gerber, D. E. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir. Med. 6, 472–478 (2018).
Khwaja, A. KDIGO clinical practice guideline for acute kidney injury. Nephron. Clin. Pract. 120, c179–c184 (2012).
Gupta, S., Cortazar, F. B., Riella, L. V. & Leaf, D. E. Immune checkpoint inhibitor nephrotoxicity: update 2020. Kidney360 1, 130–140 (2020).
Ma, Y., Wang, Q., Dong, Q., Zhan, L. & Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 9, 1546–1553 (2019).
Ferrara, R. et al. Do immune checkpoint inhibitors need new studies methodology? J. Thorac. Dis. 10, S1564–S1580 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Nishino, M. et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin. Cancer Res. 19, 3936–3943 (2013).
Pignon, J.-C. et al. irRECIST for the evaluation of candidate biomarkers of response to nivolumab in metastatic clear cell renal cell carcinoma: analysis of a phase II prospective clinical trial. Clin. Cancer Res. 25, 2174–2184 (2019).
Seymour, L. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18, e143–e152 (2017).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Lee, J. H. et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 4, 717–721 (2018).
Okada, H. et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 16, e534–e542 (2015).
Di Giacomo, A. M. et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol. Immunother. 58, 1297–1306 (2009).
Masuhiro, K., Shiroyama, T., Nagatomo, I. & Kumanogoh, A. Unique case of pseudoprogression manifesting as lung cavitation after pembrolizumab treatment. J. Thorac. Oncol. 14, e108–e109 (2019).
Tanizaki, J. et al. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer 102, 44–48 (2016).
Hochmair, M. J., Schwab, S., Burghuber, O. C., Krenbek, D. & Prosch, H. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer 113, 4–6 (2017).
Kohorst, M. A. et al. Transfusion reactions in pediatric and adolescent young adult haematology oncology and immune effector cell patients. EClinicalMedicine 26, 100514 (2020).
Kim, J., Park, J. H. & Shin, S. Effectiveness of simulation-based nursing education depending on fidelity: a meta-analysis. BMC Med. Educ. 16, 152 (2016).
Okuda, Y. et al. The utility of simulation in medical education: what is the evidence? Mt. Sinai J. Med. 76, 330–343 (2009).
Azoulay, E., Shimabukuro-Vornhagen, A., Darmon, M. & von Bergwelt-Baildon, M. Critical care management of chimeric antigen receptor T cell-related toxicity. Be aware and prepared. Am. J. Respir. Crit. Care Med. 200, 20–23 (2019).
r ICU resource utilization in critically ill patients following chimeric antigen receptor T-cell therapy. Am. J. Respir Crit. Care Med. 202, 1184–1187 (2020).
Redelman-Sidi, G. et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors). Clin. Microbiol. Infect. 24, S95–S107 (2018).
Davies, H. D. & Committee on Infectious Diseases. Infectious complications with the use of biologic response modifiers in infants and children. Pediatrics 138, e20161209 (2016).
Hill, J. A. & Seo, S. K. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 136, 925–935 (2020).
Fishman, J. A., Hogan, J. I. & Maus, M. V. Inflammatory and infectious syndromes associated with cancer immunotherapies. Clin. Infect. Dis. 69, 909–920 (2019).
Yakoub-Agha, I. et al. Management of adults and children undergoing CAR T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 105, 297–316 (2019).
Kelly, D. F. et al. Plasma and memory B-cell kinetics in infants following a primary schedule of CRM 197-conjugated serogroup C meningococcal polysaccharide vaccine. Immunology 127, 134–143 (2009).
Perez, E. E. et al. Update on the use of immunoglobulin in human disease: a review of evidence. J. Allergy Clin. Immunol. 139, S1–S46 (2017).
Tomblyn, M. et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol. Blood Marrow Transpl. 15, 1143–1238 (2009).
Hu, Y. et al. CAR T-cell treatment during the COVID-19 pandemic: management strategies and challenges. Curr. Res. Transl. Med. 68, 111–118 (2020).
Bisogno, G. et al. Clinical characteristics and outcome of severe acute respiratory syndrome coronavirus 2 infection in Italian pediatric oncology patients: a study from the Infectious Diseases Working Group of the Associazione Italiana di Oncologia e Ematologia Pediatrica. J. Pediatric Infect. Dis. Soc. 9, 530–534 (2020).
Mohammadi, A., Esmaeilzadeh, E., Li, Y., Bosch, R. J. & Li, J. Z. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. EbioMedicine 59, 102903 (2020).
Zhou, X., Zhou, J. & Zhao, J. Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect. Dis. 20, 500 (2020).
Ruark, J. et al. Patient-reported neuropsychiatric outcomes of long-term survivors after chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transpl. 26, 34–43 (2020).
Broome, H. E., Rassenti, L. Z., Wang, H. Y., Meyer, L. M. & Kipps, T. J. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk. Res. 35, r–1394 (2011).
Mueller, K. T. et al. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin. Cancer Res. 24, 6175–6184 (2018).
Weissert, R. Progressive multifocal leukoencephalopathy. J. Neuroimmunol. 231, 73–77 (2011).
Brown, P. et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc Netw. 18, 81–112 (2020).
Maude, S. L. et al. Efficacy of humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in children and young adults with relapsed/refractory acute lymphoblastic leukemia [abstract]. Blood. 128, 217 (2016).
Maude, S. L. et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL [abstract]. J. Clin. Oncol. 34, 3011 (2016).
Lanza, F. et al. CD22 expression in B-cell acute lymphoblastic leukemia: biological significance and implications for inotuzumab therapy in adults. Cancers 12, 303 (2020).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Shah, N. N. et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 38, 1938–1950 (2020).
Neelapu, S. S. et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat. Rev. Clin. Oncol. 15, 218 (2018).
Dufner, V. et al. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 3, 2491–2498 (2019).
Lund, C. C. The estimation of areas of burns. Surg. Gynecol. Obste 79, 352–358 (1944).
Acknowledgements
We thank the MD Anderson Cancer Center CAR T Cell Therapy-Associated Toxicity (CARTOX) Program, The University of Texas MD Anderson Cancer Center, the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Hematopoietic Cell Transplantation-Cancer Immunotherapy (HCT-CI) Subgroup and the PALISI Network Scientific Committee, the Supportive Care Committee of the Pediatric Transplantation and Cellular Therapy Consortium (PTCTC), the Extracorporeal Life Support Organization (ELSO) and the Paediatric Diseases Working Party (PDWP) of the European Society of Blood and Marrow Transplantation (EBMT). D.R., S.J.K., D.McC., B.C., B.S., J.M., P.T., D.P., F.N.H.T., P.K., K.R., S.S.N, E.J.S. and K.M.M. are members of the CARTOX Program. H.A, A.H.A., M.D.N., B.S., M.E.S. and K.M.M. are members of the PALISI HCT–CI subgroup. H.A.A., J.A., S.W.C. and K.M.M are members of the PTCTC. M.D.N. is a member of ELSO. S.C. is a member of the PDWP of the EBMT. We thank our patients and families who inspire and guide us towards continuous improvement. We thank our respective nursing unit staffs and key stakeholders who work to ensure access to novel therapies for our patients. C.M.R. is the recipient of a K23 grant (1K23HL150244) from the National Heart, Lung and Blood Institute.
Author information
Authors and Affiliations
Contributions
D.R., S.J.K.,H.A., D.McC., A.H.A., B.C., C.G., L.C., B.S., R.D.S., E.J.S. and K.M.M. wrote the manuscript. All authors made meaningful contributions to and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.G. has served on the advisory board for Janssen and Legend Biotech. A relative of J.B.G. is a consultant to Acceleron and Celgene. K.R. and E.J.S. have a licence agreement with Takeda. E.J.S. has served on the advisory boards of Adaptimmune, Axio, Bayer, Celgene, Magenta, Mesoblast and Novartis. K.M.M. serves as a site investigator for, receives research funding from and has served as a medical consultant for Atara Biotherapeutics, and has received research and medical education funds from and served as a medical consultant for Jazz Pharmaceuticals. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks B. Geoerger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Foundation for the Accreditation of Cellular Therapy: http://www.factwebsite.org
US NIH ClinicalTrials.gov database: https://www.clinicaltrials.gov
Supplementary information
Rights and permissions
About this article
Cite this article
Ragoonanan, D., Khazal, S.J., Abdel-Azim, H. et al. Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nat Rev Clin Oncol 18, 435–453 (2021). https://doi.org/10.1038/s41571-021-00474-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-021-00474-4
This article is cited by
-
Metabolically hijacked Listeria monocytogenes synergizes STING activation and dual cell death for enhanced antitumor immunotherapy
Journal of Nanobiotechnology (2025)
-
Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy
Leukemia (2024)
-
Riding the storm: managing cytokine-related toxicities in CAR-T cell therapy
Seminars in Immunopathology (2024)
-
Acute lung pathology in the immunocompromised child
Pediatric Radiology (2024)
-
lnsights into Adjuvant Systemic Treatment Selection for Patients with Stage III Melanoma: Data from the Dutch Cancer Registry
Targeted Oncology (2024)