Abstract
Cyclin-dependent kinase (CDK) 4/6 inhibition in combination with endocrine therapy is the standard-of-care treatment for patients with advanced-stage hormone receptor-positive, HER2 non-amplified (HR+HER2−) breast cancer. These agents can also be administered as adjuvant therapy to patients with higher-risk early stage disease. Nonetheless, the clinical success of these agents has created several challenges, such as how to address acquired resistance, identifying which patients are most likely to benefit from therapy prior to treatment, and understanding the optimal timing of administration and sequencing of these agents. In this Review, we describe the rationale for targeting CDK4/6 in patients with breast cancer, including a summary of updated clinical evidence and how this should inform clinical practice. We also discuss ongoing research efforts that are attempting to address the various challenges created by the widespread implementation of these agents.
Key points
-
Cyclin-dependent kinase (CDK) 4/6 inhibitors inhibit the cell cycle, thus inducing cellular senescence and are increasingly being implicated in antitumour immunity.
-
Three CDK4/6 inhibitors are currently used in routine clinical practice and have shown highly consistent progression-free survival results, although inconsistencies have emerged in their overall survival results.
-
The toxicity profile varies between different CDK4/6 inhibitors, allowing a degree of treatment tailoring based on the needs of individual patients.
-
The increased and earlier use of CDK4/6 inhibitors has resulted in a better understanding of the mechanisms of acquired resistance; potential treatment combinations that might overcome these mechanisms are being explored.
-
Treatment selection after disease progression on a CDK4/6 inhibitor remains an area of active research and is likely to be influenced by the underlying mechanisms of resistance
Similar content being viewed by others
Introduction
Aberrant progression through the cell cycle is an established hallmark of cancer and various stages of this cycle have become attractive targets for the development of anticancer drugs1. In this regard, the development of the cyclin-dependent kinase 4 (CDK4) and CDK6 inhibitors, of which three (palbociclib, ribociclib and abemaciclib) have become widely clinically implemented over the past 8 years2,3,4, has been a major success. Use of these agents in patients with metastatic hormone receptor-positive (HR+) breast cancer has dramatically improved clinical outcomes, and in October 2021 abemaciclib was also granted FDA approval for use in the adjuvant setting5. However, the increased and earlier use of these drugs is beginning to create another set of problems, including the development of acquired resistance and what to do once patients have disease progression on one of these agents. Our understanding of other ways in which CDK4/6 inhibition might affect cancer growth, in particular by altering the tumour immune microenvironment, is expanding and has led to both preclinical investigations and clinical trials combining CDK4/6 inhibitors with immune-modulating anticancer drugs.
At a time when the use of CDK4/6 inhibitors in patients with breast cancer is rapidly expanding, we aim to summarize the established mechanism of action of these agents, and then discuss their more recently described immunological effects. We summarize the latest data from phase III trials testing CDK4/6 inhibitors in both the metastatic and adjuvant settings in patients with HR+ breast cancer, including data on overall survival (OS), and discuss the possible reasons for the differences in outcomes seen between the agents. We also review the use of CDK4/6 inhibitors in patients with other breast cancer subtypes as well as the mechanisms of resistance to these drugs and the various novel approaches being explored in both preclinical studies and early phase clinical trials in an attempt to overcome this challenge.
CDK4/6 and the cell cycle
Aberrant cellular proliferation, resulting from dysregulation of the processes controlling cell division, is one of the hallmarks of cancer1. Halting this rapid division is a key aim of anticancer therapeutic agents. The cell cycle is controlled by a tightly regulated system of checkpoints and processes that must be passed in a stepwise fashion for the cell to divide successfully6. CDKs that become activated through interactions with their partner cyclins7,8 and regulate specific transitions are a key part of this process.
A longstanding interest exists in CDKs as potential targets for anticancer drugs, although data from early trials testing pan-CDK inhibitors were generally negative, principally owing to substantial toxicities9,10. These adverse events are thought in part to be mediated by inhibition of CDK1, in light of the observation that Cdk1 knockout is embryonically lethal in mouse models. Thus, identifying cancers that are dependent on individual CDK isozymes for proliferation and increasing the selectivity of the inhibitors8 has been key to the development of safe and effective CDK inhibitors.
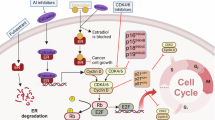
The kinases CDK4 and CDK6 share several structural and functional similarities11, and mediate transition through the restriction point in G1, principally through phosphorylation of the cell cycle repressor protein retinoblastoma 1 (RB1). Cells that are dependent on CDK4/6 for proliferation typically have low expression levels of D-type cyclin (cyclins D1–D3) during G0 and early G1. As the level of D type cyclins increases, the resultant cyclin D–CDK4/6 complex partially phosphorylates RB1 (hypophosphorylation) as well as the RB pocket proteins p130 and p107. In an unphosphorylated state, RB1 represses the transcription of genes involved in cell cycle progression via binding to the E2F transcription factor family12,13,14. RB1 phosphorylation reduces the affinity of RB1 for E2F and reduces the extent to which RB1 is able to suppress E2F function and thus disinhibits cell cycle progression15,16,17. The partially activated E2F proteins arising from binding with phosphorylated RB1 initiate the S phase transcription programme including the expression of E-type cyclins, which form complexes with CDK2 and other proteins with an important role in S phase18. A positive feedback loop is formed whereby cyclin E–CDK2 further phosphorylates RB1, thus further supressing the inhibitory signals mediated by E2F and triggering S phase transition (Fig. 1).
a, Unphosphorylated retinoblastoma 1 (RB1) inhibits the transcription of cell cycle-related genes via binding with E2F transcription factors. As the cell progresses through G0 to early G1, cyclin D levels increase and cyclin D forms complexes with cyclin-dependent kinase (CDK) 4/6. b, The cyclin D–CDK4/6 complex phosphorylates RB1, thus reducing the inhibitory effects of RB1 on E2F-mediated transcription. Partially activated E2Fs activate the S phase transcription programme, which includes expression of CCNE1/cyclin E. Cyclin E binds with and partially activates CDK2, which leads to further phosphorylation of RB1 in late G1 phase. Full activation of cyclin E/CDK2 is inhibited by p27, and proteosome-mediated degradation of p27 is the switch that triggers S phase entry and the initiation of DNA replication.
CDK regulation is affected by two groups of inhibitory proteins known as the INK family and the CDK inhibitor 1–kinase inhibitory protein (CIP/KIP) family19. The INK family comprises four structurally related proteins (p16, p15, p18 and p19)20,21,22, with a high affinity for CDK4 and CDK6 relative to other CDK isozymes20. p16 is the most widely characterized, and robust p16 upregulation is a hallmark of loss of RB1 function23,24. The p16 protein forms a complex with CDK4 and CDK6 and inhibits the binding of cyclin D, thus preventing RB1 phosphorylation and cell cycle progression25. The CIP/KIP group of proteins comprises p27, p21 and p57 (refs. 26,27,28,29). p21 and p27 are ubiquitously expressed, whereas p57 is only expressed by a subset of tissues29. p27 binds to the CDK4/6–cyclin D complex, with the trimeric complex required for full CDK4/6 activity30. In vitro, the binding of p27 to CDK4/6–cyclin D reduces the potency of clinical CDK4/6 inhibitors30, although whether this interaction affects clinical efficacy remains uncertain. By contrast, p27 inhibits CDK2 and the degradation of p27 is a key step in triggering the G1–S phase transition19,31 (Fig. 1).
The dominant role of CDK4/6 in control of the G1–S phase transition of the cell cycle is highly cell-type-dependent. Individual Cdk4 or Cdk6 mouse knockouts are non-lethal32,33,34, reflecting a large degree of functional compensation between CDK4 and CDK6. However, dual knockout of both Cdk4 and Cdk6 results in severe, fatal anaemia in the late embryonic stage, although the proliferation of non-haematological cell types is largely unaffected, with CDK2 compensating for CDK4/6 (ref. 33). Data from individual cell tracking experiments demonstrate that the level of CDK2 expression in daughter cells immediately after mitosis determines which cells become quiescent (low CDK2 activity) and which cells directly progress through the cell cycle (high CDK2 activity)35. Cells ‘born’ with high levels of CDK2 activity probably do not require CDK4/6 for proliferation, and this observation might explain the lack of activity of CDK4/6 inhibitors in tumours derived from such cells36.
CDK4/6 in breast cancer
Successful inhibition of CDK4/6 signalling in cancer relies on an intact cyclin D–CDK4/6–RB1 axis, and cell-type-specific dependence on CDK4/6 in order to transition through the G1 restriction point. Cyclin D1 is often expressed at high levels in samples obtained from patients with oestrogen receptor-positive (ER+) breast cancer37,38,39. In approximately 13% of primary breast cancers, this overexpression reflects amplification of CCND1, which encodes cyclin D1 (ref. 40). Furthermore, CCND1 expression is directly regulated by the ER41; however, high levels of cyclin D1 expression can occur in the absence of CCND1 amplifications. Data from several studies indicate that cyclin D is also able to directly promote ER activation in a hormone-independent manner42,43, and these multiple links between ER signalling and cyclin D1 probably explain the selective dependence of ER+ breast cancer cells on CDK4/6 for cell cycle progression. Intact RB1 is also necessary for dependence on CDK4/6 signalling44, with RB1 inactivation occurring in only 1–2% of primary and untreated metastatic ER+ breast cancers45. RB1 loss is more frequently associated with luminal B type biology46, and might be acquired as a mechanism of resistance to CDK4/6 inhibitors.
Role of CDK4/6 inhibitors in antitumour immunity
As well as having a role in cell cycle regulation, the ability of CDK4/6 inhibitors to modulate the immune tumour microenvironment is becoming increasingly clear. CDK4/6 inhibition promotes antitumour immunity by increasing the antigen-presenting capacity of tumour cells47, increasing T cell activation48 with suppression of regulatory T (Treg) cells47, and altering the T cell balance towards memory T cells49. Data from various preclinical studies indicate the potential of CDK4/6 inhibitors to improve the efficacy of immune-checkpoint inhibitors (ICIs)47,48,50. Upregulation of genes associated with inflammation and antitumour immunity was also seen in an analysis of biopsy samples and surgical specimens obtained from patients receiving neoadjuvant abemaciclib plus anastrozole in the neoMONARCH study51.
Activation of endogenous retroviral retrotransposons by CDK4/6 inhibitors is thought to result in increased antigen presentation by tumour cells47. DNA methyltransferase 1, which suppresses such retrotransposons, is an E2F pathway target gene that is downregulated by CDK4/6 inhibition. Retrotransposon activation results in increased intracellular double-stranded RNA levels, thus triggering antiviral defence mechanisms and resulting in the upregulation of genes encoding MHC and IFN signalling47. Upregulation of genes involved in interferon signalling was also observed in biopsy samples obtained from patients receiving palbociclib in the neoadjuvant NeoPalAna trial47.
CDK4/6 inhibitors might also promote the cytotoxic T cell response. CDK4/6 inhibition in PD-1-overexpressing Jurkat T cells in vitro activates the NFAT family of proteins, resulting in IL-2 production and the proliferation, homing and activation of effector T cells in mouse models48. Expression of the chemokines CXCL9 and CXCL10, which govern trafficking of T cells to tumours52, is increased after exposure to CDK4/6 inhibitors, which perhaps explains the increased homing of T cells seen in this context48. CDK4/6 inhibition also seems to shift T cells towards a central memory phenotype (with suppression of the Treg phenotype)49, which might enable a more sustained antitumour immune response48. The higher expression of CDK6 noted in Treg cells compared with other T cell subtypes might explain the increased sensitivity of this cell type to CDK4/6 inhibitors48.
Comparing clinically approved CDK4/6 inhibitors
All of the three main CDK4/6 inhibitors approved for clinical use in Europe, the USA and several other locations2,4,53, along with dalpiciclib, which is approved in China54,55, have been designed to be relatively selective for CDK4/6 with varying degrees of inhibitory activity against other CDKs. Palbociclib has similar levels of affinity for both CDK4 and CDK6 (ref. 56), with ribociclib and abemaciclib both being more potent inhibitors of CDK4 (refs. 57,58). Ribociclib has the highest relative affinity for CDK4 versus CDK6 (ref. 59). Abemaciclib also differs from ribociclib and palbociclib in its ability to inhibit a wider range of other kinases including CDK2, albeit to a lesser degree than CDK4 or CDK6 (ref. 60). These varying affinities for CDK4 and CDK6 might explain some of the differences in the toxicity profiles of these three drugs. CDK4 is the dominant kinase in ER+ breast cancer cells, with inhibition of CDK4 thought to explain the efficacy of CDK4/6 inhibitors in this context, whereas CDK6 is the dominant kinase in bone marrow progenitors, with inhibition of CDK6 thought to result in neutropenia.
Weak inhibition of CDK2 by abemaciclib might be an important determinant of the differential activity of this agent. For example, in some in vitro models palbociclib-resistant cells are also resistant to ribociclib but remain sensitive to abemaciclib60. This apparent lack of resistance might result from concurrent CDK2 inhibition60. However, this result has not been replicated across all models61 and remains an area of active research.
Differences also seem to exist in the extent of central nervous system (CNS) penetration of abemaciclib relative to the other CDK4/6 inhibitors. In xenograft models, abemaciclib is able to cross the blood–brain barrier (BBB) with enhanced levels of penetration compared with those of palbociclib62. This enhanced penetration probably reflects reduced efflux of abemaciclib compared with that of palbociclib owing to differing interactions with the efflux transporters present on the BBB62. CNS penetration of abemaciclib in sufficient concentrations to enable clinically relevant inhibition of CDK4/6 has been confirmed in clinical studies63. Other clinically relevant differences between these drugs include variations in bioavailability and metabolism, such that abemaciclib requires twice daily dosing owing to a shorter elimination half-life (18.3 h versus 29 h for palbociclib and 32 h for ribociclib).
CDK4/6 inhibitors in patients with HR+ breast cancer
Metastatic breast cancer
Three CDK4/6 inhibitors have been approved in Europe and/or the USA as well as in several other locations, for clinical use in combination with endocrine therapy in patients with metastatic HR+HER2− breast cancer: palbociclib, ribociclib and abemaciclib. These three inhibitors have demonstrated remarkably consistent median progression-free survival (PFS) outcomes64 (Table 1). Both palbociclib and abemaciclib have also received FDA approval for use in men with breast cancer65,66.
Palbociclib
Results from PALOMA-1 (ref. 67), a randomized phase II study testing palbociclib plus letrozole in the first-line setting, led to the accelerated FDA approval of this combination in 2015 based on a statistically significant improvement in median PFS2. This improvement was confirmed in the subsequent phase III PALOMA-2 study68 (Table 1). In both studies, all patients had received no previous treatment for metastatic disease and were not permitted to have received any prior chemotherapy.
The phase III PALOMA-3 trial69,70 demonstrated the superiority of fulvestrant plus palbociclib versus fulvestrant plus placebo (Table 1). All patients had had disease progression on prior endocrine therapy and were permitted to have received up to one line of chemotherapy for advanced-stage disease. As well as a significant improvement in median PFS, patients receiving fulvestrant plus palbociclib had a longer median time to subsequent chemotherapy (17.9 versus 8.8 months).
Abemaciclib
In the first-line setting, data from the phase III MONARCH-3 trial demonstrated similar results to those achieved with palbociclib plus letrozole with the combination of an aromatase inhibitor (anastrozole or letrozole) plus abemaciclib71 (Table 1). Almost half of the patients (46.7%) had received prior endocrine therapy for early stage breast cancer, including 27% who had previously received an aromatase inhibitor. Benefit was observed across all subgroups including those who previously received an aromatase inhibitor.
In the phase III MONARCH-2 trial72, the combination of fulvestrant plus abemaciclib was tested in patients with disease progression on first-line endocrine therapy or disease relapse on adjuvant endocrine therapy. Only one prior line of endocrine therapy was permitted in the metastatic setting and no prior chemotherapy. A significant improvement in PFS was observed in the abemacicilb-treated cohort versus those receiving placebo as well as a significantly increased objective response rate (ORR) of 48.1% versus 21.3% with an aromatase inhibitor alone in participants with measurable disease (Table 1).
In the phase II MONARCH-1 study73 single-agent abemaciclib was tested in patients with advanced-stage HR+HER2− breast cancer. The trial population was heavily pretreated (median three lines of prior therapy) although all participants had not previously received a CDK4/6 inhibitor. An ORR of 19.7% was reported as well as a median PFS of 6 months. This ORR is higher than those seen in a small phase II study testing single-agent palbociclib (ORR 6%)74 and a phase I study testing ribociclib (ORR 5.6%)75. These patients were also heavily pretreated, with ≥70% having received two or more prior lines of treatment and the other 30% receiving four or more lines prior to study entry. This increased efficacy might reflect the weak inhibition of CDK2 seen with abemaciclib but not palbociclib or ribociclib, although this remains an unproven hypothesis given that abemaciclib also inhibits a range of other kinases. The only CDK4/6 inhibitor to receive FDA approval for use as a single agent is abemaciclib. This agent can be used as monotherapy in patients with advanced HR+HER2− breast cancer who have progressed on endocrine therapy and chemotherapy, although it is still not approved outside the USA4. This approval was based on data from the MONARCH-1 trial.
Ribociclib
The phase III MONALEESA-2 trial76 was designed to investigate the efficacy of ribociclib plus letrozole in the first-line setting. Median PFS data are consistent with those seen with both abemaciclib and palbociclib in similar cohorts (Table 1).
In the phase III MONALEESA-3 trial77, the efficacy of ribociclib plus fulvestrant was compared with that of fulvestrant, resulting in a significant improvement in median PFS (Table 1). Approximately half of the cohort had previously received endocrine therapy for advanced-stage disease with the other half having disease relapse on adjuvant endocrine therapy. The numerically higher median PFS in the treatment arm of the MONALEESA-3 trial (20.5 months) relative to the PALOMA-3 trial (9.5 months) probably reflects the fact that around half of the patients in the MONALEESA-3 trial were receiving study treatment in the first line. Indeed, data from a subgroup analysis demonstrate that patients receiving second-line therapy in the treatment arm of the MONALEESA-3 trial had a numerically lower median OS than those receiving first-line therapy78.
Ribociclib is the only CDK4/6 inhibitor assessed in a cohort comprising specifically premenopausal patients in the MONALEESA-7 trial57,79, although other studies have also included such patients (Table 1). All participants were prescribed ovarian function suppression regardless of endocrine backbone. No prior endocrine therapy was permitted for patients with advanced-stage disease, although up to one previous line of chemotherapy was allowed. Median PFS and OS in this population are consistent with those from studies involving postmenopausal patients. In July 2018, the FDA-approved indication for ribociclib in combination with an aromatase inhibitor was expanded to include premenopausal and perimenopausal patients. This expansion did not permit combination with tamoxifen owing to the increased risk of cardiotoxicity53. A general consensus exists that CDK4/6 inhibitors have the same level of activity in premenopausal patients receiving ovarian suppression as that seen in postmenopausal patients.
Toxicities
Several clinically important differences in toxicity profile exist between the three widely approved CDK4/6 inhibitors. In phase I safety evaluations, neutropenia was the main dose-limiting toxicity (DLT) seen with palbociclib and ribociclib whereas ribociclib also resulted in other DLTs including QTc interval prolongation75,80,81, which was more frequent in those receiving tamoxifen as endocrine therapy in the MONALEESA-7 trial (in 16% versus 7% of patients)79. By contrast, the main DLTs seen with abemaciclib were diarrhoea and fatigue82.
These results are reflected in the phase III data, in which the most frequent toxicity seen with both palbociclib and ribociclib was neutropenia (grade 3–4 events in 66.5% of patients versus 1.4% with placebo in the PALOMA-2 trial68, and 60% versus 4% in the MONALEESA-2 trial68,83). However, very low incidences of febrile neutropenia were reported with both drugs (2.1% with palbociclib and 1.5% with ribociclib), probably indicating that a subset of neutrophils remained functional despite lower circulating levels. Other common toxicities seen with both drugs include fatigue, nausea and alopecia. Ribociclib seems to lead to a greater incidence of nausea of any grade (in 53% versus 35% of patients) and of liver function test abnormalities (20% versus 12%) than palbociclib68,76. In the MONALEESA-2 trial, ten patients receiving ribociclib (3%) had QTc interval prolongation of >60 ms compared with one patient (0.3%) in the letrozole group76. Most of these patients were asymptomatic, and the events were managed using ribociclib dose modifications; nonetheless, current guidelines suggest that all patients commencing ribociclib should undergo electrocardiographic monitoring for the first two cycles84. The concomitant prescription of QT-prolonging medication is also contraindicated.
In contrast to palbociclib and ribociclib, abemaciclib has a more distinct toxicity profile. Diarrhoea occurred in up to 90% of patients receiving abemaciclib in the MONARCH-2 and MONARCH-3 trials, and lead to dose alterations (omission, reduction or discontinuation) in 20%85. Ribociclib-induced diarrhoea typically occurs early in the course of treatment with a median time of onset of 7 days. However, the incidence of grade 3–4 neutropenia in the abemaciclib cohort of the MONARCH-3 trial (in 24% of patients86) was lower than that seen with other CDK4/6 inhibitors. Another adverse effect that seems to be unique to abemaciclib is an increase in serum creatinine, which generally persists for the duration of treatment. This effect seems to reflect a reduction in urinary creatinine excretion without any detectable change in renal function, which returns to previous levels on discontinuation of abemaciclib. A small but definite increased risk of venous thromboembolism (VTE) has also been noted with abemaciclib. In the MONARCH-3 trial, 20 patients (6.1%) developed VTE versus 0.6% of those receiving placebo71. In a real-world analysis of data from 364 patients receiving abemaciclib, 7.4% developed a thrombotic event87. In the MONARCH-E trial, the risk of VTE was further increased by the combination of abemaciclib with tamoxifen (4.3% versus 1.8% with combination with an aromatase inhibitor)88. This increased risk is an important consideration in premenopausal patients who are more likely to receive tamoxifen and should be carefully balanced according to individual patient-specific risk factors for thromboembolism.
Postmarketing surveillance studies have revealed an increased risk of pneumonitis or interstitial lung disease in patients receiving any one of the three drugs compared with patients in control arms (1.6% versus 0.7%)89. However, the mechanisms through which these events develop are not yet understood.
These differences in toxicity profiles provide clinicians with the opportunity to tailor the choice of medication to best suit the needs of individual patients. In the event of problematic toxicities with one agent, switching to an alternative CDK4/6 inhibitor is generally considered a reasonable option.
OS outcomes
Data on OS are now available for the three approved CDK4/6 inhibitors. In contrast to PFS data, OS data are not consistent across agents (Table 1). For ribociclib, longer-term follow-up revealed consistent statistically significant improvements in OS both in combination with fulvestrant in the MONALEESA-3 trial90 and with letrozole in MONALEESA-2 (ref. 91). A similar level of improvement was also seen in premenopausal patients in the MONALEESA-7 trial92. For abemaciclib, long-term follow-up also revealed a statistically significant improvement both in combination with fulvestrant in the MONARCH-2 trial93, and a numerically similar although not yet statistically significant improvement in combination with letrozole in the MONARCH-3 trial94. By contrast, palbociclib conferred a numerical but not statistically significant improvement in OS in combination with fulvestrant in the PALOMA-3 trial95, but showed no clear evidence of improved OS in combination with letrozole in the PALOMA-1 trial96 or the PALOMA-2 trial97. Given the similar median PFS data, these results are somewhat surprising, with a number of possible explanations.
For the studies testing fulvestrant-containing combinations, differences in study design perhaps provide the most likely explanation for the differences in OS outcomes. The cohort of the PALOMA-3 trial included a greater percentage of heavily pretreated patients (>50% of patients had received two or more prior lines of therapy and 33% had received chemotherapy for advanced-stage disease), which reduced the efficacy of fulvestrant in the control arm, and therefore also limited the extent of absolute improvement in median PFS (6.8 months). Inclusion criteria for the MONARCH-2 trial allowed patients to have received a single prior line of endocrine therapy, and resulted in an intermediate absolute improvement in PFS (8.5 months), whereas the MONALEESA-3 trial largely recruited patients with disease relapse on adjuvant endocrine therapy, and demonstrated the greatest absolute improvement in median PFS (12.2 months). The OS results match the absolute improvement in PFS, with the MONALEESA-3 trial demonstrating the greatest median OS benefit and the PALOMA-3 trial the lowest. A potential conclusion from the fulvestrant studies, is that CDK4/6 inhibitors should predominantly be used in the first-line setting, in which the drugs provide the greatest level of absolute benefit, and that this ultimately translates into the most substantial improvements in OS.
For studies testing a CDK4/6 inhibitor plus letrozole, two distinct interpretations exist. Firstly, these studies were not designed with sufficient power to detect a statistically significant improvement in OS, and this limited power increased the risk of any findings occurring by chance, even though the true effect on OS was the same. In the PALOMA-2 trial, statistical power was likely to have been further reduced by 27% of patients crossing over to a CDK4/6 inhibitor following disease progression, and a lack of OS data from 13% of patients in the palbociclib arm and 21% in the placebo arm, which might have resulted in bias. The highly consistent median PFS results from these trials support this view as the most likely explanation for the lack of an improvement in median OS seen with palbociclib.
The second interpretation of data from the studies involving letrozole is that a genuine difference in the efficacy of the agents exists, with palbociclib being less efficacious than either ribociclib or abemaciclib as reflected in the lack of a significant improvement in OS that was somehow not captured in the PFS results. Preclinical data suggesting that abemaciclib and ribociclib are both more potent inhibitors of CDK4 than of CDK6 (refs. 57,58), with CDK6 inhibition resulting in greater bone marrow-related adverse events98 and thus potentially limiting the tolerability of palbociclib, provide support for this view.
Current use of CDK4/6 inhibitors
Based on the studies described previously, CDK4/6 inhibitors are now recommended as first-line therapies in the majority of patients with advanced-stage HR+HER2− breast cancer. Exceptions to this recommendation that preclude the use of CDK4/6 inhibitors in certain patients, include incipient organ failure and/or other relevant comorbidities such as poor liver function. In patients with incipient organ failure (typically owing to aggressive disease at risk of visceral crisis with symptomatic organ infiltration and rapidly progressing disease) the standard approach has been to use chemotherapy, followed by maintenance endocrine therapy and a CDK4/6 inhibitor, although a meeting abstract presented in March 2023 from the phase II RIGHT Choice trial provides some evidence that ribociclib plus endocrine therapy could be more effective than chemotherapy in this setting99. The recommended companion endocrine therapy for a CDK4/6 inhibitor is an aromatase inhibitor, or fulvestrant if the patient had disease progression on, or within 12 months of, stopping such therapy in the adjuvant setting. The age and menopausal status of the patient should not influence decision making100.
The choice of CDK4/6 inhibitor partly reflects physician preference, although the differences noted between the three approved drugs allow for some matching of toxicity profiles with comorbidities. Palbociclib has been the preferred standard choice for many physicians largely owing to the earlier approval of this agent, although a shift towards the use of other CDK4/6 inhibitors has occurred following the availability of OS data.
The correct timing of administration for CDK4/6 inhibitors (first-line versus later-line) was addressed in the SONIA trial101. Patients were randomly assigned to receive first-line palbociclib plus an aromatase inhibitor followed by second-line fulvestrant versus an aromatase inhibitor as first-line therapy followed by second-line palbociclib plus fulvestrant, with no difference in time to second progression (HR 0.87, 95% CI 0.74–1.03; P = 0.10). This study was underpowered to evaluate differences in OS101. On the surface, the SONIA trial challenges the standard clinical paradigm of giving an aromatase inhibitor plus a CDK4/6 inhibitor as first-line therapy, although it is important to note that this study was designed to show superiority of the CDK4/6 inhibitor combination in the first-line rather than non-inferiority. Since the SONIA trial was conceived, single-agent fulvestrant has been surpassed and is now a suboptimal second-line therapy after an aromatase inhibitor plus a CDK4/6 inhibitor as its activity is too low102,103,104; furthermore, palbociclib is also no longer the preferred CDK4/6 inhibitor for many physicians. Several more-effective alternatives to single-agent fulvestrant are now available in the second-line setting: in patients with ESR1 mutations elacestrant provides a more effective alternative102, as does fulvestrant plus alpelisib combination therapy in patients with PIK3CA and/or AKT signalling pathway alterations105 and, subject to approval, capivasertib104 and everolimus combinations. The SONIA trial probably opens up the possibility of administering aromatase inhibitors as single-agent first-line therapy, perhaps in older patients with comorbidities, although these data are unlikely to widely affect clinical practice.
Adjuvant therapy
A substantial majority of patients with breast cancer present with early stage disease (stage I–III) and can be offered potentially curative treatment in the form of surgery with or without neoadjuvant and/or adjuvant chemotherapy and/or radiotherapy. Endocrine therapy typically given for 5–10 years has led to reduced relapse rates, although a substantial number of patients still have disease relapse with either local or distant recurrences106. Several large trials have assessed the potential of CDK4/6 inhibitors to prolong recurrence-free survival in the adjuvant setting in both premenopausal and postmenopausal women. In contrast with the similar outcomes of trials involving patients with metastatic disease, clear differences in efficacy seem to exist in this setting.
Palbociclib
The randomized phase III PALLAS trial107 compared 2 years of palbociclib plus endocrine therapy versus endocrine therapy alone following primary surgery in 5,761 patients with stage II–III ER+HER2− breast cancer. The choice of a 2-year course of palbociclib in this study was based on a number of factors including the cytostatic mechanism of action of palbociclib suggesting the need for extended therapy, as evidenced by extended use of endocrine therapy for up to 10 years following primary breast cancer treatment108,109, and the ability to safely and effectively monitor patients for adverse events. Endocrine therapy was continued for 5–10 years according to the standard-of-care approach. The primary end point was invasive disease-free survival (iDFS). After 4 years of follow-up monitoring, iDFS was 84.2% in the palbociclib group versus 84.5% in the placebo group. The incidence of toxicities was substantially higher in the palbociclib group (grade 3 and 4 adverse events in 67.5% and 5.7% of patients, respectively, versus 14.4% and 0.8% in the placebo group), and were mostly haematological, consistent with the experience in trials involving patients with advanced-stage disease (Table 2).
A smaller-cohort phase III study, the PENELOPE-B trial110, also assessed the efficacy of palbociclib in the adjuvant setting, albeit in a higher-risk population who had residual disease after neoadjuvant chemotherapy. This study enrolled 1,250 women with a clinical pathological staging–ER grading (CPS-EG) score of \(\ge \)3 or \(\ge \)2 with positive lymph nodes. Participants were randomly assigned to receive 1 year of palbociclib plus standard endocrine therapy versus standard endocrine therapy plus placebo. No statistically significant difference in the primary end point of iDFS emerged between the groups after a median follow-up duration of 42.8 months (Table 2). A small absolute OS benefit of 4.3% emerged at 2 years, which was lost with further follow-up. The incidence and type of toxicities observed was similar to that seen in the PALLAS trial and with palbociclib in the metastatic setting.
Abemaciclib
In contrast to the PALLAS and Penelope-B trials, the MONARCH-E trial demonstrated both substantial and statistically significant clinical benefit with the addition of abemaciclib to standard endocrine therapy in patients with ER+HER2− high-risk early stage breast cancer111. In this randomized trial, participants received continuous abemaciclib for 2 years alongside standard-of-care endocrine therapy or endocrine therapy alone. This cohort included 5,637 patients with high-risk disease defined as four or more tumour-positive lymph nodes or one to three positive lymph nodes with one of the following additional features: tumour diameter >5 cm, poorly differentiated (grade ≥3) tumours or Ki67 expression on >20% of tumour cells. iDFS at 2 years was 92.2% in the abemaciclib plus endocrine therapy arm and 88.7% in the endocrine therapy plus placebo arm. An updated analysis at a median follow-up duration of 42 months showed an increase in the extent of benefit from abemaciclib with a 4-year iDFS of 85.5% in the abemaciclib arm versus 78.6% in the endocrine therapy arm112 (Table 2). This result provides clear evidence that abemaciclib continues to provide clinical benefit after patients have completed the prespecified 2-year course of treatment, a carryover effect that is also observed with adjuvant endocrine therapy. Fewer deaths occurred among patients receiving abemaciclib, although final OS data remain immature. Treatment discontinuation rates were similar across both arms (18.2% in the abemaciclib arm versus 17.1% in the endocrine therapy alone arm). In the abemaciclib arm, adverse events were the most frequent cause of treatment discontinuation (35% versus 6%), whereas non-fatal iDFS events (50% versus 30% in the abemaciclib arm) were the most frequent causes of discontinuation in the control group. Many patients (43.6%) required abemaciclib dose reductions to manage adverse effects, predominantly diarrhoea. Reassuringly, these dose reductions do not seem to have affected oncological outcomes113 and, interestingly, over half of all patients who discontinued treatment had not previously had a dose reduction88. These observations highlight the clinical importance of both appropriate patient counselling to encourage early reporting of adverse effects and the proactive management of toxicities, such as diarrhoea with loperamide, and dose reductions to enable longer-term treatment compliance.
Data from the MONARCH-E trial also demonstrated that Ki67 has prognostic value (in that high Ki67 index scores are associated with a worse prognosis), although the relative level of benefit from abemaciclib was the same in patients with high and low Ki67 index scores112. In October 2021, abemaciclib received FDA approval as adjuvant therapy in patients with early stage HR+HER2− breast cancer meeting the same criteria for high-risk disease as those with advanced-stage disease114. The requirement for a Ki67 index of ≥20% was removed in an updated approval announcement from March 2023 (ref. 115).
Ribociclib
Preliminary data from the phase III NATALEE trial were reported in June 2023 (ref. 116). A total of 5,101 patients with stage ≥IIA HR+HER2− breast cancer were enrolled and randomly assigned 1:1 to ribociclib or placebo plus endocrine therapy. This cohort is a somewhat lower-risk population than that of the MONARCH-E trial103. NATALEE also included a small number of male patients (0.4%). Ribociclib was administered at a dose of 400 mg daily (lower than the standard dose of 600 mg daily used in patients with metastatic disease) in order to improve tolerability in the adjuvant setting. At the latest cut-off, 19% of patients had discontinued ribociclib owing to adverse events. In this trial, ribociclib was given for up to 3 years, extending the duration of therapy beyond that seen in other studies, in an attempt to maximize efficacy. Toxicities were consistent with those found in studies testing ribociclib in the metastatic setting. The NATALEE trial met its primary end point of improved iDFS, with a 3-year iDFS of 90.4% versus 87.1% translating into an absolute benefit of 3.3% with ribociclib (Table 2).
At the time of writing, the NATALEE trial has had substantially shorter follow-up than the MONARCH-E trial with only 20% of patients having completed 3 years of treatment at the latest cut-off. Despite this incomplete follow-up, the improvement in outcomes is lower than that reported in the latest update from the MONARCH-E trial. This difference might reflect the broader inclusion criteria applied in the NATALEE trial, which included patients with lower-risk disease. In the NATALEE trial, 20% of the cohort had stage IIA disease and 12% had node-negative disease after pathology review (28% were clinically node-negative).
Longer-term results from the NATALEE trial are needed to draw definitive conclusions on the efficacy of adjuvant ribociclib in patients with lower-risk, stage II disease and whether any benefit is maintained following completion of, or discontinuation of treatment. Pending regulatory assessment, ribociclib might provide an option for lower-risk patients, in particular those who do not meet the inclusion criteria for the MONARCH-E trial and are therefore unable to receive abemaciclib. OS data from the NATALEE trial remain immature.
Explaining the divergent adjuvant results
Given the similarities in the primary end points of phase III studies testing CDK4/6 inhibitors in the metastatic setting, the divergent results of the PALLAS and MONARCH-E trials were initially surprising; however, several possible reasons for these discrepant results exist. The MONARCH-E trial recruited higher-risk patients, and the more favourable outcomes seen in this study might simply reflect the fact that patients with higher-risk disease, as seen in those with other breast cancer subtypes including those with HER2+ disease in the APHINITY trial117, derive greater benefit from adjuvant therapy. However, a subgroup analysis of data from the PALLAS trial did not reveal greater benefit in high-risk patients107. The Penelope-B trial110 also recruited higher-risk patients than the PALLAS trial, although patients received only 1 year of adjuvant palbociclib, and a longer treatment duration might have yielded different results.
Compliance in the PALLAS trial was relatively poor, with 42% of patients discontinuing treatment before the end of the planned 2 years of palbociclib, and this percentage was substantially lower in the MONARCH-E trial (18%)112 perhaps reflecting greater tolerance of toxicity in the higher-risk cohort.
Differences in activity between the drugs provide the most plausible explanation for the differences in outcomes between the studies. Abemaciclib monotherapy has a higher response rate in heavily pretreated patients with endocrine therapy-resistant disease than either palbociclib or ribociclib73,75,118, although this difference in efficacy is no longer apparent when the drugs are combined with endocrine therapies in earlier lines of therapy. Early disease relapse in the adjuvant setting probably reflects the existence of a subpopulation of highly proliferative cancers119, which are considered to be either insensitive or minimally sensitive to endocrine therapy, and data from this group potentially more closely matches those from the metastatic setting120. Although unproven, the ability to inhibit a broader range of kinases, particularly CDK2, might explain the increased single-agent activity of abemaciclib in such cancers. At this time, comparing the results of the MONARCH-E and NATALEE trials, enabling comparisons of abemaciclib and ribociclib, is inadvisable owing to the limited follow-up on the NATALEE trial.
Neoadjuvant therapy
Neoadjuvant endocrine therapy has historically been reserved for a small number of patients; nonetheless, interest in the activity of CDK4/6 inhibitors in early stage breast cancer has prompted the initiation of several trials investigating the combination of a CDK4/6 inhibitor plus endocrine therapy in the neoadjuvant setting. All of the neoadjuvant studies thus far have been phase II randomized trials and can be divided into those comparing a CDK4/6 inhibitor plus endocrine therapy with chemotherapy, such as the NeoPAL121,122 and CORALLEEN123 trials, and those with endocrine therapy alone as the control arm, such as the PALLET124, FELINE125, MONALEESA-1 (ref. 126) and neoMONARCH51 trials. The end points chosen vary but include biological markers of response and biomarkers thought to be associated with outcomes such as change in Ki-67 index score and pathological complete response (pCR) rates.
In the two studies comparing CDK4/6 inhibitors plus endocrine therapy with chemotherapy, high-risk patients were selected using the same 50-gene expression profiling assay122,126. In both studies, all patients had either intermediate or high genomic risk scores at baseline. The NeoPAL trial (palbociclib plus endocrine therapy versus three cycles of 5-fluorouracil–epirubicin–cyclophosphamide followed by three cycles of docetaxel) failed to meet its primary end point of 20% of patients with a residual cancer burden score of 0–1. Notably, outcomes in the control arm were also poor in this trial with only 15% of patients having a residual cancer burden score of 0–1, which might reflect the intrinsic resistance of HR+ breast cancer to chemotherapy. In the CORALLEEN trial postmenopausal women with HR+ luminal B disease were randomized to ribociclib plus endocrine therapy or chemotherapy. The primary end point was change in risk of recurrence score from baseline to surgery. The numbers of patients with changes in risk of recurrence score were broadly similar between the groups and, although ribociclib plus endocrine therapy clearly had some activity, it did not outperform chemotherapy. The studies comparing a CDK4/6 inhibitor plus endocrine therapy with endocrine therapy only generally showed higher antiproliferative effects with the combination, albeit with no significant differences in pCR. Longer-term follow-up on event-free survival in all of the studies discussed in this section is currently awaited.
One of the key barriers to the implementation of CDK4/6 inhibitors in the neoadjuvant setting is the lack of availability of accurate biomarkers enabling the identification of patients who are most likely to benefit from the addition of a CDK4/6 inhibitor, as many patients have excellent outcomes with endocrine therapy alone122. In the single-arm, phase II DxCARTES trial127, responses to a neoadjuvant CDK4/6 inhibitor plus endocrine therapy and molecular downstaging seemed to be independent of baseline tumour biology, and further research is needed to identify effective biomarkers.
Other breast cancer subtypes
Positive data on the activity of CDK4/6 inhibitors in patients with HR+ breast cancer have led to interest in testing these agents in other tumour types. As a heterogeneous group, triple-negative breast cancers (TNBCs) have been considered resistant to CDK4/6 inhibitors, in part owing to aberrant cyclin E1 expression in basal-like TNBCs128 as well as more frequent alterations in RB1 (ref. 45). However, the subclassification of TNBCs by gene expression patterns129 has revealed a luminal androgen receptor (LAR) subtype that expresses the androgen receptor (AR). Cell lines and mouse xenograft mouse models reflecting the LAR subtype have been shown to respond to palbociclib36. A phase I trial testing this agent in combination with the anti-PD-1 antibody avelumab in patients with AR+ TNBC is currently ongoing (NCT04360941).
HER2+ breast cancers are also often of the luminal subtype, with mouse models harbouring Erbb2-driven tumours known to be sensitive to CDK4/6 inhibitors130,131. Preclinical evidence indicates that CDK4/6 inhibitors might counteract resistance to HER2-targeted therapies, although clinical confirmation is awaited132. The combination of the HER2-targeted therapies trastuzumab and pertuzumab plus palbociclib is being investigated in the neoadjuvant setting (NA-PHER trial) and the available data thus far indicate a statistically significant decrease in Ki67 index (geometric mean 31.9 at baseline versus 12.1 at time of surgery; P = 0.013) as well as a promising pCR rate (27%)133,134. This combination appears tolerable and could provide a chemotherapy-free option for patients with HER2+ breast cancer given the similar ORR to that seen with chemotherapy in the NeoSphere trial135. In the metastatic setting, three clinical trials are currently investigating the combination of a CDK4/6 inhibitor plus a HER2-targeted therapy and endocrine therapy. The phase III PATINA trial (NCT02947685) aims to determine the effect of adding palbociclib to maintenance therapy with trastuzumab or pertuzumab plus endocrine therapy following induction chemotherapy. Recruitment has now finished, and the study is expected to complete in 2026. The phase II PATRICIA trial (NCT02448420) is evaluating the use of palbociclib alongside trastuzumab with or without letrozole in a heavily pretreated population of patients with HER2+ breast cancer (who have received two or more prior lines of therapy including trastuzumab). Early analysis of data from cohorts A and B (comprising patients with a luminal intrinsic subtype) demonstrated longer PFS in those receiving the triplet136. This study has now closed to recruitment. Elsewhere, the MONARCH-HER trial randomized women with advanced-stage HR+, HER2+ disease to abemaciclib plus fulvestrant and trastuzumab (group A), abemaciclib plus trastuzumab (group B) or standard-of-care chemotherapy plus trastuzumab (group C). These patients were heavily pretreated including trastuzumab in 96%. This study met its primary end point of a difference in PFS between group A and group C (8.3 versus 5.7 months)137. Median OS, although numerically longer at 31.1 months in arm A versus 20.7 months in arm C, failed to reach statistical significance (HR 0.75, 95% CI 0.47–1.21; P = 0.24), possibly reflecting a lack of statistical power138. Follow-up of this cohort is currently ongoing, and this combination could provide a chemotherapy-free option for patients with advanced-stage HER2+ disease.
CDK4/6 inhibitors plus ICIs
The combination of chemotherapy plus the anti-PD-L1 antibody atezolizumab139 or the anti-PD-1 antibody pembrolizumab140 is now approved as a standard-of-care therapy for patients with PD-L1+ metastatic TNBC, although the role of these agents in patients with HR+ cancer is currently less clear. The majority of HR+ cancers are presumed to be ‘immunologically cold’, with low levels of tumour-infiltrating lymphocytes, thereby rendering them insensitive to ICIs. Given the immunomodulatory effects of CDK4/6 inhibitors, combining these with ICIs might have the potential to unlock the durable antitumour effects of the latter in a previously resistant population.
A phase Ib study testing pembrolizumab in combination with abemaciclib showed a clinical benefit rate comparable to that achieved with CDK4/6 inhibitor monotherapy in patients who are not selected for PD-L1 status141. Patients were divided into a treatment-naive cohort (cohort 1) and a previously treated cohort (cohort 2). Grade ≥3 treatment-emergent adverse events occurred in 69.2% and 60.7% of patients in cohorts 1 and 2, respectively, including two deaths related to adverse events141. In another early phase study involving patients with metastatic disease, complete response rates of up to 31% have been reported with the addition of pembrolizumab to palbociclib with median PFS and OS durations of 25.2 months and 36.9 months, respectively142. Final results from these studies will help to guide future trial planning.
Data from the PACE trial143, in which disease progression in patients receiving a CDK4/6 inhibitor plus endocrine therapy was an inclusion criterion, were reported in December 2022 and provide perhaps the most encouraging result in this area thus far. This study evaluated both continuation of palbociclib beyond disease progression as well as the addition of avelumab. Although median PFS did not change with continuation of palbociclib relative to endocrine therapy alone (4.6 versus 4.8 months), it almost doubled to 8.1 months in the triplet arm (palbociclib, avelumab and fulvestrant).
Biomarker analysis
As treatment paradigms become more complex, substantial interest has emerged in the prospective identification of patients who are most likely to derive maximum benefit from CDK4/6 inhibitors, and those whose tumour might be intrinsically resistant to therapy. This approach would enable expensive treatment to be given only to patients who are likely to derive meaningful benefit and avoid toxicities in those who are unlikely to have an improved survival outcome.
Genetic variants and intrinsic resistance
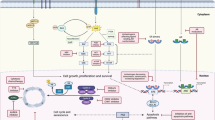
Consistent benefit from CDK4/6 inhibitors has been seen in patients with tumours both with and without common genetic alterations, such as mutations in PIK3CA and TP53 as well as CCND1 amplifications. Rare mutant subgroups of ER+ tumours exist, such as those harbouring mutations in RB1 or genes encoding other signalling pathway components, that are probably intrinsically resistant to CDK4/6 inhibitors. Tumours that are homozygous for inactivating mutations or deletions in RB1 have been resistant to treatment in preclinical models45,130 (Fig. 2) and patients with these alterations detected in circulating tumour DNA (ctDNA) derived considerably less benefit from ribociclib than those with wild-type RB1 (median PFS 3.8 versus 18.9 months) in an analysis of samples from the MONALEESA trial cohorts144. Nonetheless, pre-existing inactivating RB1 mutations or deletions are rare in untreated patients with HR+ breast cancer (<4% of patients)45,145. Furthermore, single-copy RB1 loss, with retention of the wild-type allele, is unlikely to result in resistance to therapy146, although such alterations might increase the likelihood of acquired resistance through loss of the remaining wild-type allele. The BRCA2 locus lies in close proximity to RB1 on chromosome 13. Tumours from patients with germline BRCA2 mutations typically feature deletion of the wild-type BRCA2 allele along with the neighbouring RB1 allele. The shorter PFS durations of patients with germline BRCA2 mutations have been suggested to reflect this codeletion, which leaves the remaining wild-type RB1 allele vulnerable to on-treatment mutations147.
Several mechanisms of resistance to cyclin-dependent kinase (CDK) 4/6 signalling have been established, including: inactivating mutations and/or deletions of RB1; amplification of AURKA; alterations that lead to inhibition of protein retinoblastoma 1 (RB1) phosphorylation by cyclin D–CDK4/6, including activating mutations in the genes encoding receptor tyrosine kinases (RTK), such as FGFR1 and HER2 overexpression, some of these effects might be reversible using specific RTKs; mutations and/or deletions of FAT1, ARID1A or PTEN, leading to CDK6 overexpression; CCNE1 overexpression leading to high levels of cyclin E and bypass activation of CDK2; or ESR1 mutations, which enable the oestrogen receptor (ER) to promote cellular proliferation despite CDK4/6 inhibition. AURKA, Aurora kinase A; E, oestrogen.
FAT1, a tumour suppressor gene that interacts with the Hippo signalling pathway, is rarely inactivated in HR+ breast cancer148. The presence of inactivating mutations has, however, been associated with inferior responses to CDK4/6 inhibitors149. FAT1 mutations result in increased CDK6 expression secondary to downregulation of genes encoding Hippo signalling pathway components149. Identifying truly inactivating FAT1 mutations is challenging owing to their rarity, thus limiting the incorporation of this evidence into clinical practice.
Gene expression signatures
Patients with luminal A or B breast cancer consistently derive benefit from CDK4/6 inhibitors regardless of subtype150,151, although results from the HER2-enriched population might be more divergent, with a retrospective exploratory biomarker analysis showing benefit from ribociclib but not palbociclib in this subgroup152. Whether this observation is a chance finding arising from a retrospective analysis remains unclear; nonetheless, in the ongoing phase III HARMONIA trial (NCT05207709) patients with HER2-enriched ER+ disease are being randomly allocated to receive either palbociclib or ribociclib combined with endocrine therapy153, and this trial should provide some additional clarity. The previously mentioned retrospective analysis of data from the phase III MONALEESA trial152 demonstrated that only patients with the basal-like subtype did not derive benefit from ribociclib. Tumours of this subtype have higher levels of cyclin E1 expression and gene expression patterns more in keeping with a TNBC phenotype. Patients with the rare ER+ basal-like subtype might not benefit from a CDK4/6 inhibitor, although insufficient evidence exists to support withholding treatment on this basis.
Two related gene expression signatures are associated with partial resistance to CDK4/6 inhibitors: high levels of CCNE1 expression and signatures characteristic of high levels of E2F activation. In an analysis of tissue samples obtained from patients enrolled in the PALOMA-3 trial, high levels of CCNE1 mRNA correlated with an inferior response to palbociclib (median PFS 7.6 versus 14.1 months)154. Cyclin E overexpression can induce resistance by activating CDK2, enabling bypass signalling155. In preclinical studies, cells overexpressing cyclin E were particularly sensitive to CHK1 inhibitors156, WEE1 inhibition157 and PKMYT1 inhibition158. Biomarker analysis of samples from neoadjuvant trials (NeoPalAna and neoMONARCH) demonstrated an association between gene expression signatures associated with IFN signalling and resistance to CDK4/6 inhibitors159. Nonetheless, the clinical applicability of all of these findings is uncertain as some activity, albeit at lower levels, was observed in patients with gene expression signatures associated with resistance.
Biomarkers of benefit from CDK4/6 inhibitors in the adjuvant setting remain elusive. Thus far, no biomarker analysis from the MONARCH-E or NATALEE trial cohorts has been reported; however, data from the PENELOPE-B and PALLAS trials presented in March 2023 suggest a composite biomarker incorporating the intrinsic subtype, Progesterone receptor status and ERBB2 (encoding HER2) expression that can be assessed in surgical specimens that might enable the identification of a subgroup of patients likely to derive the greatest benefit from adjuvant palbociclib160.
Mechanisms of acquired resistance
Despite CDK4/6 inhibitors being highly effective therapies, most patients ultimately develop resistance. A number of molecular alterations have been implicated in resistance to these agents161. RB1 loss is an established mechanism of resistance that has been demonstrated in both cell lines and patient-derived xenograft models162. In clinical practice, up to 10% of patients with disease progression on CDK4/6 inhibitors acquire alterations or deletions of both copies of RB1 but such alterations are not seen in patients receiving endocrine therapy alone163. RB1 alterations can be detected in ctDNA164.
Given the number of patients who now receive CDK4/6 inhibitors, the number of patients with RB1-deficient tumours will increase; therefore, identifying clinically effective therapeutic strategies for this growing subset will become increasingly important. After RB1 loss, progression through the cell cycle is driven in a CDK4/6-independent manner33. Aurora kinase A (AURKA), a kinase with an essential role in cell division, has been implicated in both intrinsic and acquired resistance to both CDK4/6 inhibitors161 and endocrine therapy165. Interestingly, inhibition of AURKA appeared to be synthetic lethal in the presence of RB1 loss in preclinical models166,167, and this effect is being explored in this clinical setting168. In preclinical models, RB1 loss seemed to be predictive of a response to targeted inhibition of the spindle assembly checkpoint threonine tyrosine kinase169.
Certain palbociclib-resistant cell lines acquire CDK6 amplifications following prolonged exposure to CDK4/6 inhibitors, with CDK6 expression promoting resistance170. High levels of CDK6 expression can also arise, at least in part following suppression of TGFβ signalling mediated by changes in microRNA expression in such cells171. Knockdown of CDK6 can restore sensitivity to CDK4/6 inhibitors170. In preclinical models, CDK6 expression was also increased by the loss of PTEN and ARID1A172. The loss of PTEN in such models could be overcome with the combination of an AKT inhibitor and a CDK4/6 inhibitor173. Data from the CAPiTello-291 trial reported in December 2022 confirmed clinical activity of the AKT inhibitor capivasertib in patients with mutations in genes encoding AKT signalling pathway components (including PTEN)174.
Defects in growth factor signalling pathways, such as FGFR1 overexpression, are another well-described mechanism of resistance to endocrine therapy175,176, and such alterations were also shown to reduce sensitivity to CDK4/6 inhibitors both preclinically177 and in an evaluation of clinical samples from the MONARCH-3 trial (ref. 178). This mechanism of resistance is thought to be mediated by alterations in the signalling pathways downstream of the ER and MAPK activation, and resistant phenotypes could be reversed using an FGFR inhibitor176. Indeed, adding the FGFR tyrosine kinase inhibitor erdafitinib to fulvestrant and palbociclib supressed tumour growth more effectively than palbociclib with or without endocrine therapy in FGFR1-overexpressing patient-derived xenograft models177. Mutations in downstream elements of the FGFR signalling pathway have also been identified in samples obtained from a cohort of patients with CDK4/6 inhibitor-resistant tumours161, providing a potential target for future treatment.
The oncogenic transcription factor c-MYC (subsequently referred to as MYC) is upregulated in CDK4/6 inhibitor-resistant cell lines179 and acquired mutations in MYC were identified in 5% of patients with disease progression on abemaciclib in the MONARCH-3 trial and 9% in the nextMONARCH trial178. MYC upregulation might therefore be another biomarker of acquired resistance.
CDK4/6 inhibitors are administered alongside endocrine therapy; therefore patients might develop resistance to the endocrine component of this treatment combination. Mutations in ESR1 are the most widely described mechanism of resistance to such agents and, although rarely detected before endocrine therapy is given45, can be found in tumours that develop resistance to endocrine therapy. An analysis of samples from the PALOMA-3 trial demonstrated the presence of several ESR1 mutations with selection of ESR1Y537S in the resistant population163. Data from the EMERALD trial published in 2022 (ref. 102) suggest that differential responses to subsequent therapy can occur in the presence or absence of this mutation102,180. Patients were enrolled following progression on a CDK4/6 inhibitor plus endocrine therapy and randomized to fulvestrant plus an aromatase inhibitor versus elacestrant, an orally administered selective ER degrader (SERD). Median PFS was significantly increased in patients with tumours harbouring ESR1 mutations receiving elacestrant (from 2.1 to 8.6 months), and this effect was more pronounced in patients with a longer duration of previous treatment with a CDK4/6 inhibitor180. Based on these data, in January 2023 the FDA approved elacestrant for use in patients with ESR1-mutated advanced-stage breast cancer with disease progression following one or more prior lines of endocrine therapy (ref. 181).
Activating mutations in ERBB2 have also been implicated in resistance to both endocrine therapy and CDK4/6 inhibitors182 and, at least according to preclinical evidence, resistance can be overcome by the addition of the HER2 tyrosine kinase inhibitor neratinib183,184. Some clinical evidence indicating the efficacy of neratinib in this setting was provided by the phase II SUMMIT study185,186.
Patients might accumulate multiple subclones and therefore harbour several different alterations that collectively contribute to a resistant phenotype. Identifying mechanisms of resistance using a precision approach, perhaps involving ctDNA tracking prior to the development of clinical resistance in the form of radiologically detectable disease recurrence, might help to reduce the proliferation of resistant clones and enable early treatment, and thus delay the onset of disease progression. In the PADA1 study, patients receiving palbociclib plus an aromatase inhibitor were monitored for ESR1 mutational status in ctDNA and randomly allocated to continuation of the aromatase inhibitor versus fulvestrant with continuation of palbociclib in the event of a mutation being detected187. Initial results suggest a benefit from treatment switching at this stage (median PFS 5.7 versus 11.9 months; HR 0.61, 95% CI 0.43–0.86; P = 0.004); final results are awaited.
Treatment after CDK4/6 inhibition
Single-agent endocrine therapy, such as fulvestrant, is less effective in patients with disease progression on a CDK4/6 inhibitor. In the CAPitello-291 trial, median PFS on fulvestrant alone was 2.6 months in patients who had previously received a CDK4/6 inhibitor versus 7.2 months in those who had not104. Regimens combining endocrine therapy plus an inhibitor of the PI3K–AKT–mTOR pathway are increasingly being seen as the standard-of-care approach following disease progression on a CDK4/6 inhibitor. Two studies have tested alpelisib, a PI3K inhibitor, in this setting: the SOLAR-1 trial (ref. 188) and the smaller BYLieve trial105. In the SOLAR-1 trial, resistance to endocrine therapy was the main inclusion criterion and only 6% of participants had previously received a CDK4/6 inhibitor. This small subgroup derived a similar level of benefit from alpelisib to the rest of the cohort. The BYLieve trial also demonstrated clinical activity of alpelisib in patients who had previously received CDK4/6 inhibitors103. Targeting other downstream elements of this signalling pathway is an attractive prospect. In the CAPitello-291 trial104, the AKT inhibitor capivasertib conferred similar levels of benefit in both the CDK4/6 inhibitor-pretreated and naive cohorts.
Elacestrant, as discussed previously, is another drug with improved efficacy compared to endocrine therapy in patients with disease progression on a CDK4/6 inhibitor102. Following FDA approval, this agent is likely to become the standard-of-care for patients with ESR1-mutated cancer following progression on a CDK4/6 inhibitor181.
Trials with results published over the past few years have investigated the potential of continued use of CDK4/6 inhibitors beyond disease progression. In the phase II MAINTAIN trial, patients with disease progression on endocrine therapy plus a CDK4/6 inhibitor were randomly allocated to receive exemestane or fulvestrant with or without ribociclib189. The majority of patients had previously received fulvestrant (83%) plus palbociclib (84%). Median PFS was 5.3 months with the addition of ribociclib to either fulvestrant or exemestane versus 2.8 months with endocrine therapy alone (HR 0.56, 95% CI 0.37–0.83; P = 0.004). Similarly, in the phase II PACE trial patients with HR+HER2− breast cancer with disease progression on a CDK4/6 inhibitor were randomly allocated to receive fulvestrant with or without palbociclib after progression190. The majority of patients (90.9%) had previously received palbociclib, and no significant difference in median PFS was observed (4.6 months with palbociclib plus endocrine therapy versus 4.8 months with endocrine therapy). The change in the type of CDK4/6 inhibitor received in the majority of the MAINTAIN trial cohort, compared with continuation of the same CDK4/6 inhibitor in the PACE trial, might explain these divergent results. In patients enrolled in the PALMIRA trial, palbociclib was also deliberately continued, despite earlier disease progression on this agent191. Patients were randomly assigned to continue palbociclib plus second-line endocrine therapy or second-line endocrine therapy alone. No significant difference in median PFS was observed (4.2 vs 3.6 months; HR 0.8, 95% CI 0.66-1.1; P = 0.206)191. For abemaciclib, data from a small observational study suggest some activity in patients who have previously received palbociclib192. In the phase III postMONARCH trial (NCT05169567), which is now closed to recruitment, eligible patients must have had disease progression on first-line endocrine therapy plus a CDK4/6 inhibitor or following adjuvant therapy, and are being randomly allocated to abemaciclib plus fulvestrant versus fulvestrant alone. This trial will provide further evidence on the efficacy of continuing with CDK4/6 inhibitors in the second-line setting. Interestingly this study also includes patients with disease progression after receiving CDK4/6 inhibitors in the adjuvant setting, which is becoming an increasingly important question given the approval of adjuvant abemaciclib in March 2023. The full results of these and further studies will be required to ascertain whether continuing treatment with CDK4/6 inhibitors beyond disease progression provides any benefit, and whether treatment sequencing is important.
Using ctDNA to guide therapy
Liquid biopsies provide a less invasive method of assessing the presence or absence of certain genomic alterations relative to analysis of tumour tissue biopsy samples, and potentially enable more sensitive monitoring for disease recurrence than conventional imaging. In the context of CDK4/6 inhibitors, two potential uses of this technology might emerge in the future. Shortly after starting CDK4/6 inhibitors, patients with a response to treatment typically have a reduction in ctDNA levels, whereas those without a response have unchanged ctDNA levels relative to baseline193. These early dynamic alterations in ctDNA levels could be used to identify patients who are likely to need additional therapy. In this regard, the phase II FAIM trial (NCT04920708), which is currently recruiting patients, is randomly assigning patients without a reduction in ctDNA level relative to baseline after 2 weeks of CDK4/6 inhibition to receive the AKT inhibitor ipatasertib plus ongoing therapy versus ongoing therapy only.
After an initial response to therapy, liquid biopsies can be used to monitor for the emergence of resistance mutations prior to the detection of disease progression on imaging. The PADA1 trial187 used liquid biopsy samples to detect the emergence of ESR1 mutations during first-line therapy with palbociclib plus an aromatase inhibitor, with patients randomly assigned to fulvestrant versus an aromatase inhibitor on detection of ESR1 mutations prior to radiological progression having substantial improvement in PFS. The phase III SERENA6 trial (NCT04964934), is recruiting patients and will monitor for ESR1 mutations in liquid biopsies from those receiving first-line CDK4/6 inhibitors, with patients switched from aromatase inhibitors to the next-generation orally administered SERD camizestrant plus continuation of the CDK4/6 inhibitor following the detection of ESR1 mutations prior to disease progression. In the future, such approaches are likely to transform how we use CDK4/6 inhibitors in the clinic with the potential to personalize therapy much more precisely than is possible currently. The use of ctDNA to monitor for early molecular relapse in patients with HR+ disease receiving adjuvant therapy is currently under evaluation in a large-cohort phase II trial (NCT04985266).
Future directions
A number of novel approaches are being investigated in an attempt to improve the efficacy of CDK4/6 inhibitors. At least some of the haematological toxicities associated with these agents are a result of CDK6 inhibition in bone marrow. Thus, CDK6 inhibition might result in toxicity, but no additional efficacy. Selective inhibitors of CDK4, with substantially reduced CDK6 inhibition, are in early phase clinical trials with early results showing some efficacy in patients with HR+ breast cancer and encouraging safety data for the combination with fulvestrant194.
Similarities in the ATP-binding sites of CDK family members present a challenge to the development of truly selective inhibitors. Nonetheless, CDK2 has been implicated as a key mediator of bypass signalling that confers resistance to CDK4/6 inhibitors and is therefore an attractive target for novel anticancer therapies195. Inhibition of CDK2 in preclinical models enhances the antitumour activity of CDK4/6 inhibitors179. Combined CDK2/4/6 inhibition, if sufficiently tolerable, therefore has the potential to provide improved efficacy. Initial results with the CDK2/4/6 inhibitor PF-3600 provide some evidence of clinical activity in patients who had previously received a CDK4/6 inhibitor, with predominant toxicities including myelosuppression196. Alternatively, selective CDK2 inhibitors have demonstrated activity in cells previously exposed to a CDK4/6 inhibitor162, and in preclinical models harbouring CDK2-activating alterations such as CCNE1 amplifications197,198. Owing to the potential for alternative CDKs to compensate for CDK2 inhibition, the selection of patients with cancers harbouring specific dependencies on CDK2 is likely to be required for successful clinical development.
Combining CDK4/6 inhibitors with other targeted therapies, such as PI3K inhibitors, is an attractive possibility. Preclinical data suggest the existence of synergy between these two therapeutic modalities, although further research is needed to identify biomarkers that are predictive of response, given these combinations have not been successful in all models162,199,200. Furthermore, a phase Ib trial testing alpelisib in combination with ribociclib and fulvestrant had to cease enrolment owing to substantial toxicities201. Nonetheless, results from a study testing palbociclib plus the PI3K inhibitor inavolisib are awaited (NCT04191499). Finally, the more widespread use of liquid biopsies is likely to dramatically alter the approach to both patient monitoring and the adjustment of treatment regimens, including CDK4/6 inhibitors, over the coming years.
Conclusions
The implementation of CDK4/6 inhibitors has dramatically changed the treatment paradigm for patients with HR+ breast cancer with substantial improvements in survival outcomes seen in both the early stage and metastatic settings. The use of these drugs is likely to expand further once results of ongoing clinical trials investigating their use both in other breast cancer subtypes and in combination with other targeted therapies and immunotherapies are published. Future priorities will include optimizing CDK4/6 inhibitor-based treatment combinations and sequences, and developing novel approaches that can overcome treatment resistance.
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Beaver, J. A. et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin. Cancer Res. 21, 4760–4766 (2015).
Shah, A. et al. FDA approval: ribociclib for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer. Clin. Cancer Res. 24, 2999–3004 (2018).
US Food and Drug Administration. FDA approves abemaciclib for HR-positive, HER2-negative breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (2017).
US Food and Drug Administration. FDA approves abemaciclib with endocrine therapy for early breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-endocrine-therapy-early-breast-cancer (2021).
Hartwell, L. H., Culotti, J., Pringle, J. R. & Reid, B. J. Genetic control of the cell division cycle in yeast. Science 183, 46–51 (1974).
Morgan, D. O. Principles of CDK regulation. Nature 374, 131–134 (1995).
Nurse, P. Finding CDK: linking yeast with humans. Nat. Cell Biol. 14, 776 (2012).
O’Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 14, 130–146 (2015).
Pavletich, N. P. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287, 821–828 (1999).
Weintraub, S. J., Prater, C. A. & Dean, D. C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature 358, 259–261 (1992).
Hiebert, S. W., Chellappan, S. P., Horowitz, J. M. & Nevins, J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 6, 177–185 (1992).
Sellers, W. R., Rodgers, J. W. & Kaelin, W. G. Jr. A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc. Natl Acad. Sci. USA 92, 11544–11548 (1995).
Kato, J., Matsushime, H., Hiebert, S. W., Ewen, M. E. & Sherr, C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7, 331–342 (1993).
Matsushime, H. et al. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71, 323–334 (1992).
Meyerson, M. & Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 14, 2077–2086 (1994).
Goodrich, D. W., Wang, N. P., Qian, Y. W., Lee, E. Y. & Lee, W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell 67, 293–302 (1991).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Hirai, H., Roussel, M. F., Kato, J. Y., Ashmun, R. A. & Sherr, C. J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell Biol. 15, 2672–2681 (1995).
Chan, F. K., Zhang, J., Cheng, L., Shapiro, D. N. & Winoto, A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol. Cell Biol. 15, 2682–2688 (1995).
Shapiro, G. I. et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 55, 505–509 (1995).
Benedict, W. F. et al. Level of retinoblastoma protein expression correlates with p16 (MTS-1/INK4A/CDKN2) status in bladder cancer. Oncogene 18, 1197–1203 (1999).
Parry, D., Mahony, D., Wills, K. & Lees, E. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol. Cell Biol. 19, 1775–1783 (1999).
Zerfass-Thome, K. et al. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol. Cell Biol. 17, 407–415 (1997).
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. The P21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
Toyoshima, H. & Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78, 67–74 (1994).
Lee, M. H., Reynisdottir, I. & Massague, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 9, 639–649 (1995).
Guiley, K. Z. et al. p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 366, eaaw2106 (2019).
Grimmler, M. et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128, 269–280 (2007).
Rane, S. G. et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet 22, 44–52 (1999).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (2004).
Rodriguez-Puebla, M. L. et al. Cdk4 deficiency inhibits skin tumor development but does not affect normal keratinocyte proliferation. Am. J. Pathol. 161, 405–411 (2002).
Spencer, S. L. et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383 (2013).
Asghar, U. S. et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin. Cancer Res. 23, 5561–5572 (2017).
Gillett, C. et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 54, 1812–1817 (1994).
Hunter, T. & Pines, J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 79, 573–582 (1994).
Kenny, F. S. et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin. Cancer Res. 5, 2069–2076 (1999).
Sherr, C. J. Cancer cell cycles. Science 274, 1672–1677 (1996).
Sutherland, R. L., Prall, O. W., Watts, C. K. & Musgrove, E. A. Estrogen and progestin regulation of cell cycle progression. J. Mammary Gland Biol. Neoplasia 3, 63–72 (1998).
Zwijsen, R. M. et al. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88, 405–415 (1997).
Neuman, E. et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell Biol. 17, 5338–5347 (1997).
Barretina, J. et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet 42, 715–721 (2010).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Herschkowitz, J. I., He, X., Fan, C. & Perou, C. M. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 10, R75 (2008).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233 (2018).
Lelliott, E. J. et al. CDK4/6 inhibition promotes antitumor immunity through the induction of T-cell memory. Cancer Discov. 11, 2582–2601 (2021).
Schaer, D. A. et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 22, 2978–2994 (2018).
Hurvitz, S. A. et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2− breast cancer. Clin. Cancer Res. 26, 566–580 (2020).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
US Food and Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer (2018).
Xu, B. et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat. Med. 27, 1904–1909 (2021).
NMPA approves AiRuiKang (dalpiciclib) in combination with fulvestrant for the treatment of relapsed/progressed HR+/HER2- advanced breast cancer. 2022-01-03. https://www.hengrui.com/en/media/detail-149.html (2022).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Tripathy, D., Bardia, A. & Sellers, W. R. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin. Cancer Res. 23, 3251–3262 (2017).
Gelbert, L. M. et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest. N. Drugs 32, 825–837 (2014).
Lallena, M. J. et al. In-vitro characterization of abemaciclib pharmacology in ER+ breast cancer cell lines [abstract]. Cancer Res. 75 (Suppl. 15), 3101 (2015).
Hafner, M. et al. Multiomics profiling establishes the polypharmacology of FDA-approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell Chem. Biol. 26, 1067–1080.e8 (2019).
Ogata, R., Kishino, E., Saitoh, W., Koike, Y. & Kurebayashi, J. Resistance to cyclin-dependent kinase (CDK) 4/6 inhibitors confers cross-resistance to other CDK inhibitors but not to chemotherapeutic agents in breast cancer cells. Breast Cancer 28, 206–215 (2021).
Raub, T. J. et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab. Dispos. 43, 1360–1371 (2015).
Tolaney, S. M. et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin. Cancer Res. 26, 5310–5319 (2020).
Gao, J. J. et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 21, 250–260 (2020).
Wedam, S. et al. FDA approval summary: palbociclib for male patients with metastatic breast cancer. Clin. Cancer Res. 26, 1208–1212 (2020).
US Food and Drug Administration. Highlights of prescribing information: VERZENIO. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf (2017).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2015).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17, 425–439 (2016).
Turner, N. C. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 373, 209–219 (2015).
Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35, 3638–3646 (2017).
Sledge, G. W. Jr. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017).
Dickler, M. N. et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin. Cancer Res. 23, 5218–5224 (2017).
DeMichele, A. et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. 21, 995–1001 (2015).
Infante, J. R. et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 22, 5696–5705 (2016).
Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 29, 1541–1547 (2018).
Slamon, D. J. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36, 2465–2472 (2018).
Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514–524 (2020).
Tripathy, D. et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 19, 904–915 (2018).
Flaherty, K. T. et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin. Cancer Res. 18, 568–576 (2012).
Schwartz, G. K. et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1). Br. J. Cancer 104, 1862–1868 (2011).
Patnaik, A. et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 6, 740–753 (2016).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375, 1738–1748 (2016).
Novartis. Straightforward ECG monitoring. KISQALI https://www.hcp.novartis.com/products/kisqali/metastatic-breast-cancer/safety/ecg-monitoring/ (2023).
Rugo, H. S. et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist 26, e53–e65 (2021).
Johnston, S. et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 5 (2019).
Watson, N. W., Wander, S. A., Shatzel, J. J. & Al-Samkari, H. Venous and arterial thrombosis associated with abemaciclib therapy for metastatic breast cancer. Cancer 128, 3224–3232 (2022).
Rugo, H. S. et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 33, 616–627 (2022).
Zhang, Y., Ma, Z., Sun, X., Feng, X. & An, Z. Interstitial lung disease in patients treated with cyclin-dependent kinase 4/6 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Breast 62, 162–169 (2022).
Slamon, D. J. et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann. Oncol. 32, 1015–1024 (2021).
Hortobagyi, G. N. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386, 942–950 (2022).
Lu, Y. S. et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2− advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin. Cancer Res. 28, 851–859 (2022).
Llombart-Cussac, A. et al. Final overall survival analysis of monarch 2: a phase 3 trial of abemaciclib plus fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer [abstract]. Cancer Res. 83 (Suppl. 5), PD13-11 (2023).
Goetz, M. P. et al. MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2-advanced breast cancer (ABC) [abstract LBA15]. Ann. Oncol. 33 (Suppl. 7), S1384 (2022).
Cristofanilli, M. et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2− ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin. Cancer Res. 28, 3433–3442 (2022).
Finn, R. S. et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 183, 419–428 (2020).
Finn, R. S. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL plus LET) versus placebo plus letrozole (PBO plus LET) in women with estrogen receptor positive/human epidermal growth factor receptor 2-negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2 [abstract]. J. Clin. Oncol. 40 (Suppl. 17), LBA1003 (2022).
Maurer, B., Brandstoetter, T., Kollmann, S., Sexl, V. & Prchal-Murphy, M. Inducible deletion of CDK4 and CDK6 – deciphering CDK4/6 inhibitor effects in the hematopoietic system. Haematologica 106, 2624–2632 (2021).
Lu, Y. S. et al. Primary results from the randomized phase II RIGHT Choice trial of premenopausal patients with aggressive HR+/HER2− advanced breast cancer treated with ribociclib + endocrine therapy vs physician’s choice combination chemotherapy [abstract]. Cancer Res. 85 (Suppl. 5), GS1-10 (2023).
Gennari, A. et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Sonke, G. S. et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC) [abstract]. J. Clin. Oncol. 41 (Suppl. 17), LBA1000 (2023).
Bidard, F. C. et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J. Clin. Oncol. 40, 3246–3256 (2022).
Juric, D. et al. Alpelisib plus endocrine therapy (ET) in patients with hormone receptor-positive (HR plus), human epidermal growth factor receptor 2-negative (HER2-), PIK3CA-mutated advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDK4/6i): biomarker analyses from the phase II BYLieve study [abstract]. Cancer Res 82 (Suppl. 4), P5-13-03 (2022).
Turner, N. et al. Capivasertib in hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 388, 2058–2070 (2023).
Rugo, H. S. et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 22, 489–498 (2021).
Pan, H. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Gnant, M. et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05BIG-14-03). J. Clin. Oncol. 40, 282–293 (2022).
Gray, R. G. et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer [abstract]. J. Clin. Oncol. 31 (Suppl. 18), 5 (2013).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Loibl, S. et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the Penelope-B trial. J. Clin. Oncol. 39, 1518–1530 (2021).
Johnston, S. R. D. et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 38, 3987–3998 (2020).
Johnston, S. R. D. et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 24, 77–90 (2023).
Rugo, H. S., Vitko, A. & Thoele, K. An overview of adverse event (AE) management for patients (pts) receiving abemaciclib [abstract]. J. Clin. Oncol. 40 (Suppl. 28), 239 (2022).
Royce, M. et al. FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J. Clin. Oncol. 40, 1155–1162 (2022).
US Food and Drug Administration. FDA D.I.S.C.O. burst edition: FDA approval of Verzenio (abemaciclib) with endocrine therapy for patients with HR-positive, HER2-negative, node-positive, early breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-verzenio-abemaciclib-endocrine-therapy-patients-hr-positive#:~:Text=On%20March%203%2C%202023%2C%20the,at%20high%20risk%20of%20recurrence (2023).
Slamon, D. et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: primary results from the phase III NATALEE trial [abstract]. J. Clin. Oncol. 41 (Suppl. 17), LBA500 (2023).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377, 122–131 (2017).
Malorni, L. et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann. Oncol. 29, 1748–1754 (2018).
Ribelles, N. et al. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res. 15, R98 (2013).
Cardoso, F. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31, 1623–1649 (2020).
Cottu, P. et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. 29, 2334–2340 (2018).
Ma, C. X. et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin. Cancer Res. 23, 4055–4065 (2017).
Prat, A. et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 21, 33–43 (2020).
Johnston, S. et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J. Clin. Oncol. 37, 178–189 (2019).
Khan, Q. J. et al. Letrozole plus ribociclib versus letrozole plus placebo as neoadjuvant therapy for ER plus breast cancer (FELINE trial) [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 505 (2020).
Curigliano, G. et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast 28, 191–198 (2016).
Llombart-Cussac, A. et al. Neoadjuvant letrozole plus palbociclib in patients (pts) with hormone receptor (HR)-positive/HER2-negative early breast cancer (EBC) with baseline Ki67 ≥20% and an Oncotype DX Breast Recurrence Score (R) result (RS) ≥18: DxCARTES [abstract]. Cancer Res. 82 (Suppl. 4), P5-16-12 (2022).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009).
Witkiewicz, A. K., Cox, D. & Knudsen, E. S. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 5, 261–272 (2014).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29, 255–269 (2016).
Gianni, L. et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol. 19, 249–256 (2018).
Gianni, L. et al. Effects of neoadjuvant trastuzumab, pertuzumab and palbociclib on Ki67 in HER2 and ER-positive breast cancer. NPJ Breast Cancer 8, 1 (2022).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Ciruelos, E. et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin. Cancer Res. 26, 5820–5829 (2020).
Tolaney, S. M. et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 21, 763–775 (2020).
André, F. et al. Final overall survival (OS) for abemaciclib plus trastuzumab +/− fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): a randomized, open-label, phase II trial [abstract LBA18]. Ann. Oncol. 33 (Suppl. 7), S1386–S1387 (2022).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Rugo, H. S. et al. Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: phase 1b study. NPJ Breast Cancer 8, 118 (2022).
Yuan, Y. et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur. J. Cancer 154, 11–20 (2021).
Goodman, A. Palbociclib/fulvestrant does not improve progression-free survival after progression on a CDK4/6 inhibitor in metastatic breast cancer. The ASCO Post https://ascopost.com/issues/january-25-2023/palbociclibfulvestrant-does-not-improve-progression-free-survival-after-progression-on-a-cdk46-inhibitor-in-metastatic-breast-cancer/ (2023).
André, F. et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J. Clin. Oncol. 38, 1009 (2020).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Knudsen, E. S. et al. Pan-cancer molecular analysis of the RB tumor suppressor pathway. Commun. Biol. 3, 158 (2020).
Safonov, A. M. et al. Allelic dosage of RB1 drives CDK4/6 inhibitor treatment resistance in metastatic breast cancer. J. Clin. Oncol. 40, 1010 (2022).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e6 (2018).
Li, Z. et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell 34, 893–905.e8 (2018).
Finn, R. S. et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin. Cancer Res. 26, 110–121 (2020).
Finn, R. S. et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: a joint analysis of PALOMA-2 and PALOMA-3 clinical trials. Breast Cancer Res. Treat. 184, 23–35 (2020).
Prat, A. et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J. Clin. Oncol. 39, 1458–1467 (2021).
Pascual, T. et al. HARMONIA SOLTI-2101/AFT-58: a head-to-head phase III study comparing ribociclib (RIB) and palbociclib (PAL) in patients (pts) with hormone receptor-positive/HER2-negative/HER2-enriched (HR+/HER2-/HER2-E) advanced breast cancer (ABC) [abstract 272TiP]. Ann. Oncol. 33 (Suppl. 7), S662 (2022).
Turner, N. C. et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 37, 1169–1178 (2019).
Dean, J. L., Thangavel, C., McClendon, A. K., Reed, C. A. & Knudsen, E. S. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 29, 4018–4032 (2010).
Sakurikar, N., Thompson, R., Montano, R. & Eastman, A. A subset of cancer cell lines is acutely sensitive to the Chk1 inhibitor MK-8776 as monotherapy due to CDK2 activation in S phase. Oncotarget 7, 1380–1394 (2016).
Chen, X. et al. Cyclin E overexpression sensitizes triple-negative breast cancer to Wee1 kinase inhibition. Clin. Cancer Res. 24, 6594–6610 (2018).
Gallo, D. et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature 604, 749–756 (2022).
De Angelis, C. et al. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin. Cancer Res. 27, 4870–4882 (2021).
Loibl, S. et al. Development and validation of a composite biomarker predictive of palbociclib + endocrine treatment benefit in early breast cancer: PENELOPE-B and PALLAS trials [abstract]. Cancer Res. 83 (Suppl. 5), PD17-05 (2023).
Wander, S. A. et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 10, 1174–1193 (2020).
Herrera-Abreu, M. T. et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res 76, 2301–2313 (2016).
O’Leary, B. et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. ery 8, 1390–1403 (2018).
Condorelli, R. et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 29, 640–645 (2018).
Opyrchal, M. et al. Aurora-A mitotic kinase induces endocrine resistance through down-regulation of ERα expression in initially ERα+ breast cancer cells. PLoS ONE 9, e96995 (2014).
Gong, X. et al. Aurora a kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 9, 248–263 (2019).
Oser, M. G. et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on aurora B kinase for survival. Cancer Discov. 9, 230–247 (2019).
Haddad, T. C. et al. Evaluation of alisertib alone or combined with fulvestrant in patients with endocrine-resistant advanced breast cancer: the phase 2 TBCRC041 randomized clinical trial. JAMA Oncol. 9, 815–824 (2023).
Soria-Bretones, I. et al. The spindle assembly checkpoint is a therapeutic vulnerability of CDK4/6 inhibitor-resistant ER+ breast cancer with mitotic aberrations. Sci. Adv. 8, eabq4293 (2022).
Yang, C. et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36, 2255–2264 (2017).
Cornell, L., Wander, S. A., Visal, T., Wagle, N. & Shapiro, G. I. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 26, 2667–2680.e7 (2019).
Li, Q. et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 12, 356–371 (2022).
Costa, C. et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 10, 72–85 (2020).
Turner, N. C. et al. Capivasertib in hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 388, 2058–2070 (2023).
Turner, N., Sharpe, R., Johnson, D., Pearson, A. & Ashworth, A. FGFR signalling drives the growth of triple negative and basal-like breast cancer cell lines both in vitro and in vivo. Cancer Res. 70, 5275–5286 (2010).
Mao, P. et al. Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER+ metastatic breast cancer. Clin. Cancer Res. 26, 5974–5989 (2020).
Formisano, L. et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER plus breast cancer. Nat. Commun. 10, 1373 (2019).
Goetz, M. P. et al. Acquired genomic alterations in circulating tumor DNA from patients receiving abemaciclib alone or in combination with endocrine therapy. J. Clin. Oncol. 38, 3519 (2020).
Pandey, K. et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers 12, 3566 (2020).
Kaklamani, V. et al. Oral elacestrant vs standard-of-care in estrogen receptor-positive, HER2-negative (ER+/HER2-) advanced or metastatic breast cancer (mBC) without detectable ESR1 mutation (EMERALD): Subgroup analysis by prior duration of CDK4/6i plus endocrine therapy (ET). J. Clin. Onc. 41 (no. 16_suppl.) 1070-1070 (2023).
US Food and Drug Administration. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer (2023).
Nayar, U. et al. Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat. Genet. 51, 207–216 (2019).
Croessmann, S. et al. Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin. Cancer Res. 25, 277–289 (2019).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Jhaveri, K. L. et al. Neratinib plus fulvestrant plus trastzuzumab (N+F+T) for hormone receptor-positive (HR+), HER2-negative, HER2-mutant metastatic breast cancer (MBC): outcomes and biomarker analysis from the SUMMIT trial [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 1028 (2022).
Ma, C. X. et al. A phase II trial of neratinib (NER) or NER plus fulvestrant (FUL) (N+F) in HER2 mutant, non-amplified (HER2mut) metastatic breast cancer (MBC): part II of MutHER [abstract]. Cancer Res. 81 (Suppl. 13), CT026 (2021).
Bidard, F. C. et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 23, 1367–1377 (2022).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Kalinsky, K. et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial [abstract]. J. Clin. Oncol. 40 (Suppl. 17), LBA1004 (2022).
Mayer, E. L. et al. Palbociclib after CDK4/6i and endocrine therapy (PACE): a randomised phase II study of fulvestrant, palbociclib and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer. 2022 San Antonia Breast Cancer Symposium. Abstract GS3-06. (8 December 2022).
Llombart-Cussac, A. et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[−]) advanced breast cancer (ABC): PALMIRA trial [abstract]. J. Clin. Oncol. 41 (Suppl. 16), 1001 (2023).
Wander, S. A. et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J. Natl Compr. Canc. Netw. https://doi.org/10.6004/jnccn.2020.7662 (2021).
Darrigues, L. et al. Circulating tumor DNA as a dynamic biomarker of response to palbociclib and fulvestrant in metastatic breast cancer patients. Breast Cancer Res. 23, 31 (2021).
Yap, T. A. et al. First-in-human first-in-class phase 1/2a study of the next generation CDK4-selective inhibitor PF-07220060 in patients (pts) with advanced solid tumors, enriched for HR+ HER2− mBC who progressed on prior CDK4/6 inhibitors and endocrine therapy [abstract]. J. Clin. Oncol. 41 (Suppl. 16), 3009 (2023).
Rizzolio, F., Tuccinardi, T., Caligiuri, I., Lucchetti, C. & Giordano, A. CDK inhibitors: from the bench to clinical trials. Curr. Drug Targets 11, 279–290 (2010).
Yap, T. et al. Abstract P5-16-06: A first-in-human phase 1/2a dose escalation/expansion study of the first-in-class CDK2/4/6 inhibitor PF-06873600 alone or in combination with endocrine therapy in patients with breast or ovarian cancer. Cancer Res. 82 (Suppl. 4), P5-16-06 (2022).
Brown, V. et al. Abstract 2306: BLU-222, an investigational, potent, and selective CDK2 inhibitor, demonstrated robust antitumor activity in CCNE1-amplified ovarian cancer models. Cancer Res. 82 (Suppl. 12), 2306 (2022).
Choi, Y. J. et al. Development of a selective CDK2-E inhibitor in CCNE driven cancers [abstract]. Cancer Res 81 (Suppl. 13), 1279 (2021).
Vora, S. R. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149 (2014).
O’Brien, N. A. et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res. 22, 89 (2020).
Tolaney, S. M. et al. Phase Ib study of ribociclib plus fulvestrant and ribociclib plus fulvestrant plus PI3K inhibitor (alpelisib or buparlisib) for HR+ advanced breast cancer. Clin. Cancer Res. 27, 418–428 (2021).
Author information
Authors and Affiliations
Contributions
L.M. and N.C.T. researched data for the manuscript and L.M. wrote the manuscript. All authors made a substantial contribution to discussions of content and edited and/or reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
L.M. and S.L. declare no competing interests. N.C.T. has acted as an adviser to Astra Zeneca, Exact Sciences, Gilead, GlaxoSmithKline, Guardant, Inivata, Lilly, Novartis, Pfizer, Relay therapeutics, Repare therapeutics, Roche/Genentech and Zentalis, and has received research funding from Astra Zeneca, Guardant Health, Inivata, Invitae, Merck Sharpe and Dohme, Natera, Personalis, Pfizer and Roche/Genentech.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C.L. Arteaga and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morrison, L., Loibl, S. & Turner, N.C. The CDK4/6 inhibitor revolution — a game-changing era for breast cancer treatment. Nat Rev Clin Oncol 21, 89–105 (2024). https://doi.org/10.1038/s41571-023-00840-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-023-00840-4
This article is cited by
-
CRYβB2 is a biomarker for poor prognosis and response to CDK4/6 inhibitors in breast cancer
Breast Cancer Research (2026)
-
Afuresertib plus fulvestrant for pretreated HR-positive, HER2-negative, advanced breast cancer: a phase Ib trial
Nature Communications (2026)
-
Remodeling the tumor dormancy ecosystem to prevent recurrence and metastasis
Signal Transduction and Targeted Therapy (2026)
-
Possible TLR-mediated immunostimulatory effect of Palbociclib in breast cancer
Molecular Biology Reports (2026)
-
Biomarkers of response to neoadjuvant palbociclib plus anastrozole in endocrine-resistant estrogen receptor-positive/HER2-negative breast cancer: a phase 2 trial
Nature Communications (2026)