Abstract
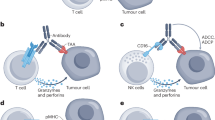
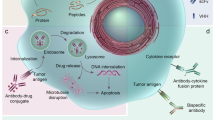
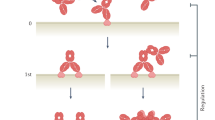
Multispecific antibodies are engineered antibody derivatives that can bind to two or more distinct epitopes or antigens. Unlike mixtures of monospecific antibodies, the binding properties of multispecific antibodies enable two specific molecules to be physically linked, a characteristic with important applications in cancer therapy. The field of multispecific antibodies is highly dynamic and expanding rapidly; to date, 15 multispecific antibodies have been approved for clinical use, of which 11 were approved for oncological indications, and more than 100 new antibodies are currently in clinical development. Nevertheless, substantial challenges limit the applications of multispecific antibodies in cancer therapy, particularly inefficient targeting of solid tumours and substantial adverse effects. Both PET and single photon emission CT imaging can reveal the biodistribution and complex pharmacology of radiolabelled multispecific antibodies. This Review summarizes the insights obtained from preclinical and clinical molecular imaging studies of multispecific antibodies, focusing on their structural properties, such as molecular weight, shape, target specificity, affinity and avidity. The opportunities associated with use of molecular imaging studies to support the clinical development of multispecific antibody therapies are also highlighted.
Key points
-
The use of multispecific antibodies in cancer treatment is expanding rapidly: 11 agents have been approved to date and many others are undergoing clinical development.
-
The design of multispecific antibodies with optimal pharmacological properties has resulted in a wide variety of molecule formats.
-
PET and single photon emission CT imaging are powerful non-invasive tools for studying the biodistribution of radiolabelled antibodies, including their uptake in tumour and healthy tissues, pharmacokinetics and target expression.
-
Preclinical and clinical molecular imaging studies of multispecific antibodies underscore key structural properties that affect their biodistribution, including target affinity and avidity, molecular weight and structure.
-
Biological factors that also influence the biodistribution of multispecific antibodies include target expression in tumour tissues, off-tumour and immune target engagement, antibody internalization, tracer dose and target saturation.
-
Molecular imaging can aid the development of multispecific antibodies by facilitating their selection and design for clinical applications and by identifying biomarkers for the stratification of patient groups.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022).
Marin-Acevedo, J. A. et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11, 39 (2018).
Jin, S. et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal. Transduct. Target. Ther. 7, 39 (2022).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. W. H. I. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug. Discov. 18, 585–608 (2019).
van de Donk, N. W. C. J. & Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 402, 142–158 (2023).
Klein, C., Brinkmann, U., Reichert, J. M. & Kontermann, R. E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug. Discov. 23, 301–319 (2024).
Goebeler, M. E., Stuhler, G. & Bargou, R. Bispecific and multispecific antibodies in oncology: opportunities and challenges. Nat. Rev. Clin. Oncol. 21, 539–560 (2024).
Przepiorka, D. et al. FDA approval: blinatumomab. Clin. Cancer Res. 21, 4035–4039 (2015).
Deshaies, R. J. Multispecific drugs herald a new era of biopharmaceutical innovation. Nature 580, 329–338 (2020).
Saez-Ibanez, A. R., Upadhaya, S. & Campbell, J. Immuno-oncology clinical trials take a turn beyond PD1/PDL1 inhibitors. Nat. Rev. Drug. Discov. 22, 442–443 (2023).
Saez-Ibañez, A. R., Sommers, E., Upadhaya, S. & Campbell, J. PD1/PDL1 clinical trials adapt to a growing landscape of patients resistant to PDx. Nat. Rev. Drug. Discov. 22, 944–945 (2023).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Stein, A. S. et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann. Hematol. 98, 159–167 (2019).
Frey, N. V. & Porter, D. L. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Am. Soc. Hematol. Educ. Program. 2016, 567–572 (2016).
Li, H., Er Saw, P. & Song, E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol. Immunol. 17, 451–461 (2020).
Sun, D., Gao, W., Hu, H. & Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B. 12, 3049–3062 (2022).
Irani, V. et al. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 67, 171–182 (2015).
Saunders, K. O. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol. 10, 1296 (2019).
Zinn, S. et al. Advances in antibody-based therapy in oncology. Nat. Cancer 4, 165–180 (2023).
Edwards, C. J. et al. The multi-specific VH-based Humabody CB213 co-targets PD1 and LAG3 on T cells to promote anti-tumour activity. Br. J. Cancer 126, 1168–1177 (2022).
Archer, S. et al. CB307: a dual targeting costimulatory Humabody VH therapeutic for treating PSMA-positive tumors. Clin. Cancer Res. 30, 1595–1606 (2024).
Power, C. A. & Bates, A. David vs. Goliath: the structure, function, and clinical prospects of antibody fragments. Antibodies 8, 28 (2019).
Suurs, F. V., Lub-de Hooge, M. N., de Vries, E. G. E. & de Groot, D. J. A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 201, 103–119 (2019).
Al Ojaimi, Y. et al. Therapeutic antibodies – natural and pathological barriers and strategies to overcome them. Pharmacol. Ther. 233, 108022 (2022).
Bensch, F. et al. Comparative biodistribution analysis across four different 89Zr-monoclonal antibody tracers – the first step towards an imaging warehouse. Theranostics 8, 4295–4304 (2018).
Lu, G. et al. Predicting therapeutic antibody delivery into human head and neck cancers. Clin. Cancer Res. 26, 2582–2594 (2020).
Dennis, M. S. et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 67, 254–261 (2007).
Mir, O. et al. Pharmacokinetics/pharmacodynamic (PK/PD) relationship of therapeutic monoclonal antibodies used in oncology: what’s new? Eur. J. Cancer 128, 103–106 (2020).
Ryman, J. T. & Meibohm, B. Pharmacokinetics of monoclonal antibodies. Clin. Pharmacokinet. 6, 493–507 (2017).
Keizer, R. J., Huitema, A. D. R., Schellens, J. H. M. & Beijnen, J. H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 49, 493–507 (2010).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Sharma, S. K. et al. Fc-mediated anomalous biodistribution of therapeutic antibodies in immunodeficient mouse models. Cancer Res. 78, 1820–1832 (2018).
Wei, W. et al. Development and characterization of CD54-targeted immunoPET imaging in solid tumors. Eur. J. Nucl. Med. Mol. Imaging 47, 2765–2775 (2020).
Zhu, M. et al. Blinatumomab, a bispecific T-cell engager (BiTE®) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin. Pharmacokinet. 55, 1271–1288 (2016).
Moek, K. L. et al. 89Zr-labeled bispecific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, heterogeneous tumor uptake. Clin. Cancer Res. 25, 3517–3527 (2019).
Park, K. et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J. Clin. Oncol. 39, 3391–3402 (2021).
European Medicines Agency. Rybrevant: product information. EMA www.ema.europa.eu/en/documents/product-information/rybrevant-epar-product-information_en.pdf (2024).
Dewulf, J., Adhikari, K., Vangestel, C., van den Wyngaert, T. & Elvas, F. Development of antibody immuno-PET/SPECT radiopharmaceuticals for imaging of oncological disorders – an update. Cancers 12, 1868 (2020).
Sneddon, D. & Cornelissen, B. Emerging chelators for nuclear imaging. Curr. Opin. Chem. Biol. 63, 152–162 (2021).
Wei, W. et al. ImmunoPET: concept, design, and applications. Chem. Rev. 120, 3787–3851 (2020).
Dammes, N. & Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 10, 938–955 (2020).
Volpe, A., Adusumilli, P. S., Schöder, H. & Ponomarev, V. Imaging cellular immunotherapies and immune cell biomarkers: from preclinical studies to patients. J. Immunother. Cancer 10, e004902 (2022).
Rowe, S. P. & Pomper, M. G. Molecular imaging in oncology: current impact and future directions. CA Cancer J. Clin. 72, 333–352 (2022).
Korpanty, G., Carbon, J. G., Grayburn, P. A., Fleming, J. B. & Brekken, R. A. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res. 13, 323–330 (2007).
Cheng, Y. A. et al. Humanized bispecific antibody (mPEG × HER2) rapidly confers PEGylated nanoparticles tumor specificity for multimodality imaging in breast cancer. J. Nanobiotechnol. 18, 118–130 (2020).
Lehmann, S. et al. In vivo fluorescence imaging of the activity of CEA TCB, a novel T-cell bispecific antibody, reveals highly specific tumor targeting and fast induction of T-cell-mediated tumor killing. Clin. Cancer Res. 22, 4417–4427 (2016).
Luo, H. et al. ImmunoPET and near-infrared fluorescence imaging of pancreatic cancer with a dual-labeled bispecific antibody fragment. Mol. Pharm. 14, 1646–1655 (2017).
Gabriëls, R. Y. et al. Fluorescently labelled vedolizumab to visualise drug distribution and mucosal target cells in inflammatory bowel disease. Gut 73, 1454–1463 (2024).
Warram, J. M. et al. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J. Pathol. Clin. Res. 2, 104–112 (2016).
Nessler, I. et al. Increased tumor penetration of single-domain antibody-drug conjugates improves in vivo efficacy in prostate cancer models. Cancer Res. 80, 1268–1278 (2020).
Oliveira, S. et al. Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Mol. Imaging 11, 33–46 (2012).
Grevys, A. et al. A human endothelial cell-based recycling assay for screening of FcRn targeted molecules. Nat. Commun. 9, 621 (2018).
Stork, R., Campigna, E., Robert, B., Müller, D. & Kontermann, R. E. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J. Biol. Chem. 284, 25612–25619 (2009).
Tijink, B. M. et al. Improved tumor targeting of anti-epidermal growth factor receptor nanobodies through albumin binding: taking advantage of modular nanobody technology. Mol. Cancer Ther. 7, 2288–2297 (2008).
Suurs, F. V. et al. Mesothelin/CD3 half-life – extended bispecific T-cell engager molecule shows specific tumor uptake and distributes to mesothelin and CD3-expressing tissues. J. Nucl. Med. 62, 1797–1804 (2021).
Waaijer, S. J. H. et al. Molecular imaging of radiolabeled bispecific T-cell engager 89Zr-AMG211 targeting CEA-positive tumors. Clin. Cancer Res. 24, 4988–4996 (2018).
Warnders, F. J. et al. Biodistribution and PET imaging of labeled bispecific T cell-engaging antibody targeting EpCAM. J. Nucl. Med. 57, 812–817 (2016).
Suurs, F. V. et al. The biodistribution of a CD3 and EpCAM bispecific T-cell engager is driven by the CD3 arm. J. Nucl. Med. 61, 1594–1601 (2020).
Hu, G. et al. Development and comparison of three 89Zr-labeled anti-CLDN18.2 antibodies to noninvasively evaluate CLDN18.2 expression in gastric cancer: a preclinical study. Eur. J. Nucl. Med. Mol. Imaging 49, 2634–2644 (2022).
Burvenich, I. J. G. et al. Radiolabelling and preclinical characterization of 89Zr-Df-radiolabelled bispecific anti-PD-L1/TGF-βRII fusion protein bintrafusp alfa. Eur. J. Nucl. Med. Mol. Imaging 48, 3075–3088 (2021).
Yu, X. et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 614, 539–547 (2023).
Mandikian, D. et al. Relative target affinities of T-cell-dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol. Cancer Ther. 17, 776–785 (2018).
Haber, L. et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci. Rep. 11, 14397 (2021).
van Lith, S. A. M. et al. Novel VHH-based tracers with variable plasma half-lives for imaging of CAIX-expressing hypoxic tumor cells. Mol. Pharm. 19, 3511–3520 (2022).
Waaijer, S. J. H. et al. Preclinical PET imaging of bispecific antibody ERY974 targeting CD3 and glypican 3 reveals that tumor uptake correlates to T cell infiltrate. J. Immunother. Cancer 8, e000548 (2020).
Kwon, L. Y., Scollard, D. A. & Reilly, R. M. 64Cu-labeled trastuzumab Fab-PEG24-EGF radioimmunoconjugates bispecific for HER2 and EGFR: pharmacokinetics, biodistribution, and tumor imaging by PET in comparison to monospecific agents. Mol. Pharm. 14, 492–501 (2017).
Razumienko, E. J., Chen, J. C., Cai, Z., Chan, C. & Reilly, R. M. Dual-receptor-targeted radioimmunotherapy of human breast cancer xenografts in athymic mice coexpressing HER2 and EGFR using 177Lu-or 111In-labeled bispecific radioimmunoconjugates. J. Nucl. Med. 57, 444–452 (2016).
Razumienko, E. J., Scollard, D. A. & Reilly, R. M. Small-animal SPECT/CT of HER2 and HER3 expression in tumor xenografts in athymic mice using trastuzumab Fab-heregulin bispecific radioimmunoconjugates. J. Nucl. Med. 53, 1943–1950 (2012).
Razumienko, E., Dryden, L., Scollard, D. & Reilly, R. M. MicroSPECT/CT imaging of co-expressed HER2 and EGFR on subcutaneous human tumor xenografts in athymic mice using 111In-labeled bispecific radioimmunoconjugates. Breast Cancer Res. Treat. 138, 709–718 (2013).
Puranik, A. D. et al. Target heterogeneity in oncology: the best predictor for differential response to radioligand therapy in neuroendocrine tumors and prostate cancer. Cancers 13, 3607 (2021).
Mileva, M. et al. Molecular imaging predicts lack of T-DM1 response in advanced HER2-positive breast cancer (final results of ZEPHIR trial). NPJ Breast Cancer 10, 4 (2024).
Sandker, G. G. W. et al. Longitudinal evaluation of the biodistribution and cellular internalization of the bispecific CD3xTRP1 antibody in syngeneic mouse tumor models. J. Immunother. Cancer 11, e007596 (2023).
Cavaliere, A. et al. Development of [89Zr]ZrDFO-amivantamab bispecific to EGFR and c-MET for PET imaging of triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 48, 383–394 (2022).
Fenis, A., Demaria, O., Gauthier, L., Vivier, E. & Narni-Mancinelli, E. New immune cell engagers for cancer immunotherapy. Nat. Rev. Clin. Oncol. 24, 471–486 (2024).
Crawford, A. et al. A mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci. Transl. Med. 11, eaau7534 (2019).
Warnders, F. J. et al. Human epidermal growth factor receptor 3-specific tumor uptake and biodistribution of 89Zr-MSB0010853 visualized by real-time and noninvasive PET imaging. J. Nucl. Med. 58, 1210–1215 (2017).
Chiu, D. et al. A PSMA-targeting CD3 bispecific antibody induces antitumor responses that are enhanced by 4-1BB costimulation. Cancer Immunol. Res. 8, 596–608 (2020).
Heynickx, N., Segers, C., Coolkens, A., Baatout, S. & Vermeulen, K. Characterization of non-specific uptake and retention mechanisms of [177Lu]Lu-PSMA-617 in the salivary glands. Pharmaceuticals 16, 692 (2023).
Abusalem, M., Martiniova, L., Soebianto, S., DePalatis, L. & Ravizzini, G. Current status of radiolabeled monoclonal antibodies targeting PSMA for imaging and therapy. Cancers 15, 4537 (2023).
Crawford, A. & Chiu, D. Targeting solid tumors using CD3 bispecific antibodies. Mol. Cancer Ther. 20, 1350–1358 (2021).
Klein, C. et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 6, e1277306 (2017).
Banushi, B., Joseph, S. R., Lum, B., Lee, J. J. & Simpson, F. Endocytosis in cancer and cancer therapy. Nat. Rev. Cancer 23, 450–473 (2023).
Chitneni, S. K., Koumarianou, E., Vaidyanathan, G. & Zalutsky, M. R. Observations on the effects of residualization and dehalogenation on the utility of N-succinimidyl ester acylation agents for radioiodination of the internalizing antibody trastuzumab. Molecules 24, 3907 (2019).
Shih, L. et al. The processing and fate of antibodies and their radiolabels bound to the surface of tumor cells in vitro: a comparison of nine radiolabels. J. Nucl. Med. 35, 899–908 (1994).
Martinez-Forero, I. et al. T cell costimulation with anti-CD137 monoclonal antibodies is mediated by K63–polyubiquitin-dependent signals from endosomes. J. Immunol. 190, 6694–6706 (2013).
Lee, J. M. et al. Novel strategy for a bispecific antibody: induction of dual target internalization and degradation. Oncogene 35, 4437–4446 (2016).
Geuijen, C. et al. A human CD137 × PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat. Commun. 12, 4445 (2021).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Bensch, F. et al. 89Zr-lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin. Cancer Res. 23, 6128–6137 (2017).
Ntzifa, A. et al. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci. Rep. 11, 2313 (2021).
Wang, Y. et al. Dose escalation PET imaging for safety and effective therapy dose optimization of a bispecific antibody. MAbs 12, 1748322 (2020).
Wijngaarden, J. E. et al. Optimal imaging time points considering accuracy and precision of Patlak linearization for 89Zr-immuno-PET: a simulation study. EJNMMI Res. 12, 54 (2022).
Menke-van der Houven van Oordt, C. W. et al. Immuno-PET imaging to assess target engagement: experience from 89Zr-anti-HER3 mAb (GSK2849330) in patients with solid tumors. J. Nucl. Med. 60, 902–909 (2019).
van de Donk, P. P. et al. Molecular imaging to support cancer immunotherapy. J. Immunother. Cancer 10, e004949 (2022).
van Brummelen, E. M. J. et al. 89Zr-labeled CEA-targeted IL-2 variant immunocytokine in patients with solid tumors: CEA-mediated tumor accumulation and role of IL-2 receptor-binding. Oncotarget 9, 24737–24749 (2018).
Sharpless, N. E. & DePinho, R. A. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug. Discov. 5, 741–754 (2006).
Bensch, F. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 24, 1852–1858 (2018).
Kist de Ruijter, L. et al. First-in-human study of the biodistribution and pharmacokinetics of 89Zr-CX-072, a novel immunoPET tracer based on an anti-PD-L1 probody. Clin. Cancer Res. 27, 5325–5333 (2021).
van Dongen, G. A. M. S. et al. The role of 89Zr-immuno-PET in navigating and derisking the development of biopharmaceuticals. J. Nucl. Med. 62, 438–445 (2021).
Kist de Ruijter, L. et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: a phase 1/2 trial. Nat. Med. 28, 2601–2610 (2022).
Morgan, P. et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug. Discov. Today 17, 419–424 (2012).
Farid, S. S., Baron, M., Stamatis, C., Nie, W. & Coffman, J. Benchmarking biopharmaceutical process development and manufacturing cost contributions to R&D. MAbs 12, 1754999 (2020).
Hemmerle, T. et al. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br. J. Cancer 109, 1206–1213 (2013).
Mårlind, J. et al. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin. Cancer Res. 14, 6515–6524 (2008).
Dakhel, S. et al. Targeted enhancement of the therapeutic window of L19-TNF by transient and selective inhibition of RIPK1-signaling cascade. Oncotarget 10, 6678–6690 (2019).
Schwager, K. et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 11, R142 (2009).
Hess, C. & Neri, D. Tumor-targeting properties of novel immunocytokines based on murine IL1β and IL6. Protein Eng. Des. Sel. 27, 207–213 (2014).
Corbellari, R. et al. A novel antibody-IL15 fusion protein selectively localizes to tumors, synergizes with TNF-based immunocytokine, and inhibits metastasis. Mol. Cancer Ther. 20, 859–871 (2021).
Pasche, N., Wulhfard, S., Pretto, F., Carugati, E. & Neri, D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin. Cancer Res. 18, 4092–4103 (2012).
Luo, H. et al. Dual targeting of tissue factor and CD105 for preclinical PET imaging of pancreatic cancer. Clin. Cancer Res. 22, 3821–3830 (2016).
Luo, H. et al. Noninvasive brain cancer imaging with a bispecific antibody fragment, generated via click chemistry. Proc. Natl Acad. Sci. USA 112, 12806–12811 (2015).
List, T. & Neri, D. Biodistribution studies with tumor-targeting bispecific antibodies reveal selective accumulation at the tumor site. MAbs 4, 775–783 (2012).
Johnson, E. E. et al. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol. Immunother. 57, 1891–1902 (2008).
Jiang, C. et al. Enhanced antitumor immune responses via a new agent [131I]-labeled dual-target immunosuppressant. Eur. J. Nucl. Med. Mol. Imaging 50, 275–286 (2023).
Koopmans, I. et al. A novel bispecific antibody for EGFR-directed blockade of the PD-1/PD-L1 immune checkpoint. Oncoimmunology 7, e1466016 (2018).
Williams, L. et al. Comparison of IL-2-antibody to IL-2-Fc with or without stereotactic radiation therapy in CEA immunocompetent mice with CEA positive tumors. Cancer Med. 13, e6909 (2024).
Gellert, J. et al. GT-00AxIL15, a novel tumor-targeted IL-15-based immunocytokine for the treatment of TA-MUC1-positive solid tumors: preclinical in vitro and in vivo pharmacodynamics and biodistribution studies. Int. J. Mol. Sci. 25, 1406 (2024).
Schilbach, K. et al. Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology 4, e1014760 (2015).
Eckert, F. et al. Enhanced binding of necrosis-targeting immunocytokine NHS-IL12 after local tumour irradiation in murine xenograft models. Cancer Immunol. Immunother. 65, 1003–1013 (2016).
The Antibody Society. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society www.antibodysociety.org/resources/approved-antibodies/ (2024).
Author information
Authors and Affiliations
Contributions
C.A.J.v.W and F.R.P. researched data for the article, wrote the manuscript and contributed substantially to discussions of its content and to review or editing of the article before submission. M.N.L.-d.H., D.J.A.d.G and E.G.E.d.V. also contributed substantially to discussions of the manuscript content and to review or editing of the manuscript before submission. A.H.B. contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.J.A.d.G. declares that he received institutional financial support for clinical trials or contracted research from Amgen, Bayer, GE Healthcare, Hoffmann La Roche and Siemens. E.G.E.d.V. declares that she received institutional financial support for acting as a member of advisory boards or consultancy services from Crescendo Biologics, Daiichi Sankyo and NSABP, as well as institutional financial support for clinical trials or contracted research grants from Amgen, Bayer, Crescendo Biologics, Genentech, Regeneron, Roche and Servier. M.N.L.-d.H. declares that she received institutional financial support for acting as a member of advisory boards or consultancy services from Merck and institutional financial support for conducting clinical and preclinical studies from Amgen, Bayer and Servier. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks Weijun Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
Primary research articles published in English from 1 January 2014 to 18 March 2024 were identified using PubMed. Search terms included the names of known multispecific antibody constructs, solid tumours and PET or SPECT radioligands. In addition, the ClinicalTrials.gov database was searched for trials of multispecific antibodies up to September 2023 and abstracts from the American Association for Cancer Research and the American Society of Clinical Oncology annual meetings from 2023 were also reviewed to identify further relevant articles. Full details of the literature search strategy are provided in the Supplementary information.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
PubMed: https://pubmed.ncbi.nlm.nih.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Winkel, C.A.J., Pierik, F.R., Brouwers, A.H. et al. Molecular imaging supports the development of multispecific cancer antibodies. Nat Rev Clin Oncol 21, 852–866 (2024). https://doi.org/10.1038/s41571-024-00946-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-024-00946-3