Abstract
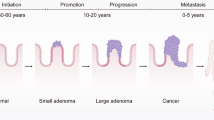
Globally, colorectal cancer (CRC) is the second leading cause of cancer death and the third most common incident cancer. CRC begins as adenomatous or serrated polyps, and in particular as advanced precursor lesions (APLs), which have the potential to progress into invasive cancers. Screening for CRC facilitates early detection and can identify cancers more amenable to cure, and can also detect and remove precursor lesions, thus also preventing CRC. Colonoscopy is the mainstay of screening in the USA and has the distinct advantage of enabling both detection and removal of precursors lesions. However, colonoscopy is burdensome, expensive and invasive, and often has negative findings. Non-invasive tests, such as testing stool samples for biomarkers of risk, have the potential to identify individuals who are more likely to benefit from colonoscopy. From a public health perspective, improving compliance with screening remains a priority. Technological innovations, including the emergence of new markers to improve stool testing and the development of blood tests that examine cell-free DNA have the potential to improve screening uptake and effectiveness. The trade-off between uptake of screening testing, detection of cancer and important precursor lesions such as APLs, and costs make for a complex calculus. In this Review, we describe the current state of CRC screening and evaluate the risks and benefits of new developments in screening.
Key points
-
On a population level, the effectiveness of colorectal cancer (CRC) screening is highly dependent on the extent of screening uptake.
-
Colonoscopy enables direct visual inspection of the entire colon and therefore provides the highest level of sensitivity and specificity for CRC screening, although this procedure is also burdensome and expensive, which limits uptake.
-
Colonoscopy facilitates CRC prevention by enabling the identification and removal of precursor lesions as part of the same procedure, whereas a positive non-invasive stool or blood test requires follow-up colonoscopy.
-
Stool-based tests offer a convenient, effective alternative to colonoscopy, although abnormal test results must be followed up with colonoscopy.
-
Blood-based assays provide sensitive methods of CRC detection but are much less sensitive for advanced precursor lesions. Owing to this limitation, blood-based tests are less effective than colonoscopy and stool-based testing for screening.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mandel, J. S. et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control study. N. Engl. J. Med. 328, 1365–1371 (1993).
Senore, C. et al. Long-term follow-up of the Italian flexible sigmoidoscopy screening trial. Ann. Intern. Med. 175, 36–45 (2022).
Holme, O. et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 312, 606–615 (2014).
Schoen, R. E. et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N. Engl. J. Med. 366, 2345–2357 (2012).
Atkin, W. et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 389, 1299–1311 (2017).
Miller, E. A., Pinsky, P. F., Schoen, R. E., Prorok, P. C. & Church, T. R. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol. Hepatol. 4, 101–110 (2019).
Hardcastle, J. D. et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 348, 1472–1477 (1996).
Sabatino, S. A. et al. Up-to-date breast, cervical, and colorectal cancer screening test use in the United States, 2021. Prev. Chronic Dis. 20, E94 (2023).
Imperiale, T. F. et al. Next-generation multitarget stool DNA test for colorectal cancer screening. N. Engl. J. Med. 390, 984–993 (2024).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Howlader N. et al. SEER cancer statistics review, 1975-2016. National Cancer Institute https://seer.cancer.gov/archive/csr/1975_2016/results_merged/topic_med_age.pdf (2019).
Ries, L. A. G. et al. SEER cancer statistics review, 1973-1999. National Cancer Institute https://seer.cancer.gov/archive/csr/1973_1999/overview.pdf (2002).
Sinicrope, F. A. Increasing incidence of early-onset colorectal cancer. N. Engl. J. Med. 386, 1547–1558 (2022).
Gupta, S., May, F. P., Kupfer, S. S. & Murphy, C. C. Birth cohort colorectal cancer (CRC): implications for research and practice. Clin. Gastroenterol. Hepatol. 22, 455–469.e7 (2024).
Lin, J. S., Perdue, L. A., Henrikson, N. B., Bean, S. I. & Blasi, P. R. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 325, 1978–1998 (2021).
Mannucci, A. et al. Colorectal cancer screening from 45 years of age: thesis, antithesis and synthesis. World J. Gastroenterol. 25, 2565–2580 (2019).
Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72, 338–344 (2023).
Islami, F. et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 68, 31–54 (2018).
Lowery, J. T. et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: a state-of-the-science review. Cancer 122, 2633–2645 (2016).
Doroudi, M., Schoen, R. E. & Pinsky, P. F. Early detection versus primary prevention in the PLCO flexible sigmoidoscopy screening trial: which has the greatest impact on mortality? Cancer 123, 4815–4822 (2017).
Segnan, N. & Armaroli, P. Early detection versus prevention in colorectal cancer screening: methods estimates and public health implications. Cancer 123, 4767–4769 (2017).
Bretthauer, M. et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 387, 1547–1556 (2022).
Sohn, E. Colonoscopies save lives. Why did a trial suggest they might not? Nature 613, 235–237 (2023).
van den Berg, D. M. N. et al. NordICC trial results in line with expected colorectal cancer mortality reduction after colonoscopy: a modeling study. Gastroenterology 165, 1077–1079.e2 (2023).
Ladabaum, U., Schoen, R. E. & Meester, R. NordICC’s 10-year interim results are unexpected and inconsistent with modeling predictions. Gastroenterology 166, 543–544 (2024).
Shaukat, A. et al. ACG clinical guidelines: colorectal cancer screening 2021. Am. J. Gastroenterol. 116, 458–479 (2021).
Cancer Research UK. Quality improvement and optimisation of bowel screening. Cancer Research UK http://www.cancerresearchuk.org/health-professional/cancer-screening/bowel-cancer-screening/quality-improvements-future-optimisation (2024).
Navarro, M., Nicolas, A., Ferrandez, A. & Lanas, A. Colorectal cancer population screening programs worldwide in 2016: an update. World J. Gastroenterol. 23, 3632–3642 (2017).
Jensen, C. D. et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann. Intern. Med. 164, 456–463 (2016).
Levin, T. R. et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology 155, 1383–1391.e5 (2018).
Doubeni, C. A. et al. Association between improved colorectal screening and racial disparities. N. Engl. J. Med. 386, 796–798 (2022).
Levin, T. R. et al. Colorectal cancer screening completion and yield in patients aged 45 to 50 years: an observational study. Ann. Intern. Med. 177, 1621–1629 (2024).
Levy, B. T. et al. Comparative performance of common fecal immunochemical tests: a cross-sectional study. Ann. Intern. Med. https://doi.org/10.7326/M24-0080 (2024).
Imperiale, T. F., Gruber, R. N., Stump, T. E., Emmett, T. W. & Monahan, P. O. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann. Intern. Med. 170, 319–329 (2019).
Senore, C. et al. Faecal haemoglobin concentration among subjects with negative FIT results is associated with the detection rate of neoplasia at subsequent rounds: a prospective study in the context of population based screening programmes in Italy. Gut 69, 523–530 (2020).
Imperiale, T. F. et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 370, 1287–1297 (2014).
Exact Sciences. New modeling data show the Cologuard® test has detected more than 623,000 cancers and precancers over past decade, saving U.S. health care system $22 billion. Exact Sciences https://www.exactsciences.com/newsroom/press-releases/new-modeling-data-show-the-cologuard-test-has-detected-more-than-623-000-cancers-and-precancers (2024).
Barnell, E. K. et al. Multitarget stool RNA test for colorectal cancer screening. JAMA 330, 1760–1768 (2023).
Imperiale, T. F., Lavin, P. & Ransohoff, D. F. Multitarget stool RNA testing. JAMA 331, 1418 (2024).
Click, B., Pinsky, P. F., Hickey, T., Doroudi, M. & Schoen, R. E. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA 319, 2021–2031 (2018).
Pinsky, P. F. et al. Number of adenomas removed and colorectal cancers prevented in randomized trials of flexible sigmoidoscopy screening. Gastroenterology 155, 1059–1068.e2 (2018).
Tutein Nolthenius, C. J. et al. Evolution of screen-detected small (6–9 mm) polyps after a 3-year surveillance interval: assessment of growth with CT colonography compared with histopathology. Am. J. Gastroenterol. 110, 1682–1690 (2015).
Vleugels, J. L. A., Hazewinkel, Y., Fockens, P. & Dekker, E. Natural history of diminutive and small colorectal polyps: a systematic literature review. Gastrointest. Endosc. 85, 1169–1176.e1 (2017).
Mohan, B. P. et al. Pooled rates of adenoma detection by colonoscopy in asymptomatic average-risk individuals with positive fecal immunochemical test: a systematic review and meta-analysis. Gastrointest. Endosc. 96, 208–222.e14 (2022).
Niedermaier, T., Seum, T., Hoffmeister, M. & Brenner, H. Lowering fecal immunochemical test positivity threshold vs multitarget stool RNA testing for colorectal cancer screening. JAMA 332, 251–252 (2024).
Brenner, H., Werner, S. & Chen, H. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 371, 184–185 (2014).
Colorectal Cancer. Use of colorectal cancer screening tests. CDC http://www.cdc.gov/colorectal-cancer/use-screening-tests/#data-table-BRFSS-Colorectal-Screening-Map-Age-Adjusted.csv (2024).
Khalid-de Bakker, C. et al. Participation in colorectal cancer screening trials after first-time invitation: a systematic review. Endoscopy 43, 1059–1086 (2011).
Inadomi, J. M. et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med. 172, 575–582 (2012).
Miller-Wilson, L. A., Rutten, L. J. F., Van Thomme, J., Ozbay, A. B. & Limburg, P. J. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a large, nationally insured cohort. Int. J. Colorectal Dis. 36, 2471–2480 (2021).
Miller-Wilson, L. A. et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a Medicaid population. Prev. Med. Rep. 30, 102032 (2022).
Jiang, L., Yang, K., Saul, M., Vajravelu, R. K. & Schoen, R. E. Multi-target stool DNA testing for colorectal cancer screening in clinical practice. Am. J. Gastroenterol. https://doi.org/10.14309/ajg.0000000000003276 (2024).
Coronado, G. D. et al. Blood-based colorectal cancer screening in an integrated health system: a randomised trial of patient adherence. Gut 73, 622–628 (2024).
Liang, P. S. et al. Blood test increases colorectal cancer screening in persons who declined colonoscopy and fecal immunochemical test: a randomized controlled trial. Clin. Gastroenterol. Hepatol. 21, 2951–2957.e2 (2023).
Centers for Medicare & Medicaid Services. Screening for colorectal cancer – blood-based biomarker tests. Report No. CAG-00454N (CMS, 2021).
Ladabaum, U., Church, T. R., Feng, Z., Ransohoff, D. F. & Schoen, R. E. Counting advanced precancerous lesions as true positives when determining colorectal cancer screening test specificity. J. Natl. Cancer Inst. 114, 1040–1043 (2022).
Chung, D. C. et al. A cell-free DNA blood-based test for colorectal cancer screening. N. Engl. J. Med. 390, 973–983 (2024).
Quinn, B. Guardant SHIELD CRC test becomes 18th ADLT test. Discoveries in Health Policy https://www.discoveriesinhealthpolicy.com/2025/03/guardant-shield-crc-test-becomes-18th.html (2025).
Shaukat, A. et al. Clinical evaluation of a blood-based screening test for the early detection of colorectal cancer. Gastroenterology 166, 1057d (2024).
Mohl, J. T. et al. Rates of follow-up colonoscopy after a positive stool-based screening test result for colorectal cancer among health care organizations in the US, 2017–2020. JAMA Netw. Open. 6, e2251384 (2023).
Corley, D. A. et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 317, 1631–1641 (2017).
San Miguel, Y., Demb, J., Martinez, M. E., Gupta, S. & May, F. P. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology 160, 1997–2005.e3 (2021).
Lee, Y. C. et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J. Natl. Cancer Inst. 109, djw269 (2017).
Levitz, C. E. et al. Using text messages and fotonovelas to increase return of home-mailed colorectal cancer screening tests: mixed methods evaluation. JMIR Cancer 9, e39645 (2023).
Sequist, T. D., Zaslavsky, A. M., Marshall, R., Fletcher, R. H. & Ayanian, J. Z. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch. Intern. Med. 169, 364–371 (2009).
Meng, W. et al. Barrier-focused intervention to increase colonoscopy attendance among nonadherent high-risk populations. World J. Gastroenterol. 15, 3920–3925 (2009).
Braschi, C. D., Sly, J. R., Singh, S., Villagra, C. & Jandorf, L. Increasing colonoscopy screening for Latino Americans through a patient navigation model: a randomized clinical trial. J. Immigr. Minor. Health 16, 934–940 (2014).
Ladabaum, U. et al. Comparative effectiveness and cost-effectiveness of colorectal cancer screening with blood-based biomarkers (liquid biopsy) vs fecal tests or colonoscopy. Gastroenterology 167, 378–391 (2024).
van den Puttelaar, R. et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 167, 368–377 (2024).
Meester, R. G., Piscitello, A., Baldo, L. & Liang, P. S. Higher assumed adherence to blood-based vs stool-based colorectal cancer screening compensates for potential lower advanced adenoma sensitivity [abstract 539]. Gastroenterology 166, S-122 (2024).
Ladabaum, U., Mannalithara, A., Schoen, R. E., Dominitz, J. A. & Lieberman, D. Projected impact and cost-effectiveness of novel molecular blood-based or stool-based screening tests for colorectal cancer. Ann. Intern. Med. https://doi.org/10.7326/ANNALS-24-00910 (2024).
Petit, J. et al. Cell-free DNA as a diagnostic blood-based biomarker for colorectal cancer: a systematic review. J. Surg. Res. 236, 184–197 (2019).
Quintero, E. et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N. Engl. J. Med. 366, 697–706 (2012).
Castells, A. et al. Effect of invitation to colonoscopy versus faecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet 405, 1231–1239 (2025).
Levin, B. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 134, 1570–1595 (2008).
Rex, D. K. et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 153, 307–323 (2017).
El Halabi, J. et al. Frequency of use and outcomes of colonoscopy in individuals older than 75 years. JAMA Intern. Med. 183, 513–519 (2023).
Deardorff, W. J. et al. Frequency of screening for colorectal cancer by predicted life expectancy among adults 76-85 years. JAMA 330, 1280–1282 (2023).
Dalmat, R. R. et al. Risk of colorectal cancer and colorectal cancer mortality beginning ten years after a negative colonoscopy, among screen-eligible adults 76 to 85 years old. Cancer Epidemiol. Biomark. Prev. 32, 37–45 (2023).
Saito, Y. et al. Colonoscopy screening and surveillance guidelines. Dig. Endosc. 33, 486–519 (2021).
Labianca, R. et al. Primary colon cancer: ESMO clinical practice guidelines for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 21, v70–v77 (2010).
Kalyta, A. et al. Association of reducing the recommended colorectal cancer screening age with cancer incidence, mortality, and costs in Canada using OncoSim. JAMA Oncol. 9, 1432–1436 (2023).
Robertson, D. J. et al. Baseline features and reasons for nonparticipation in the colonoscopy versus fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM) study, a colorectal cancer screening trial. JAMA Netw. Open. 6, e2321730 (2023).
Forsberg, A. et al. Once-only colonoscopy or two rounds of faecal immunochemical testing 2 years apart for colorectal cancer screening (SCREESCO): preliminary report of a randomised controlled trial. Lancet Gastroenterol. Hepatol. 7, 513–521 (2022).
Coronado, G. D. et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol. Biomark. Prev. 30, 2327–2333 (2021).
Selby, K. et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann. Intern. Med. 167, 565–575 (2017).
Breekveldt, E. C. H. et al. Personalized colorectal cancer screening: study protocol of a mixed-methods study on the effectiveness of tailored intervals based on prior f-Hb concentration in a fit-based colorectal cancer screening program (PERFECT-FIT). BMC Gastroenterol. 23, 45 (2023).
Chen, H. et al. Comparison of colonoscopy, fecal immunochemical test, and risk-adapted approach in a colorectal cancer screening trial (TARGET-C). Clin. Gastroenterol. Hepatol. 21, 808–818 (2023).
Lu, M. et al. Optimizing positivity thresholds for a risk-adapted screening strategy in colorectal cancer screening. Clin. Transl. Gastroenterol. 12, e00398 (2021).
Kortlever, T. L. et al. Diagnostic yield of a risk model versus faecal immunochemical test only: a randomised controlled trial in a colorectal cancer screening programme. Br. J. Cancer 129, 791–796 (2023).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.E.S. has acted as an adviser of Guardant and has received research funding from Exact Sciences, Freenome and Immunovia. M.A.McC. and A.J.M. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks H. Chen, S. Gupta and C. Senore for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCabe, M.A., Mauro, A.J. & Schoen, R.E. Novel colorectal cancer screening methods — opportunities and challenges. Nat Rev Clin Oncol 22, 581–591 (2025). https://doi.org/10.1038/s41571-025-01037-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01037-7
This article is cited by
-
Disruption of HSPA8-GEMIN5 interaction suppresses colorectal cancer by impaired splicing-translation coupling-mediated proteostasis imbalance
Journal of Experimental & Clinical Cancer Research (2026)