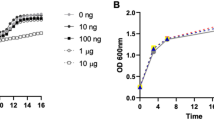
Abstract
Buruli ulcer is a slowly progressive necrotizing disease of the skin caused by Mycobacterium ulcerans, recognized by WHO as a neglected tropical disease. Around 2,000 cases are reported annually, but underdiagnosis and under-reporting probably obscure the true burden. A major advance in the understanding of Buruli ulcer pathogenesis was the discovery that mycolactone, a lipid-like exotoxin secreted by M. ulcerans, inhibits the Sec61 translocon, driving tissue destruction and immune suppression. M. ulcerans is an opportunistic environmental pathogen; however, its mechanisms of transmission remain unclear in most regions. PCR is the current gold standard for diagnosis, but its cost and technical demands limit use in resource-limited settings. Treatment is available with an oral regimen of rifampicin plus clarithromycin for 8 weeks, but further research is in progress to explore alternative drugs, optimized dosing and duration, and improved affordability. Adjunctive wound care, management of paradoxical reactions and rehabilitation, including physiotherapy and psychosocial support, are essential components of Buruli ulcer management. Future efforts should focus on elucidating transmission pathways to inform prevention, developing rapid diagnostics, refining and adapting drug regimens for diverse clinical presentations and patient groups, and advancing wound care. Strengthening healthcare worker training and integrating Buruli ulcer control with that of other skin diseases will enhance accessibility to early diagnosis and treatment, prevent disabilities and deformities, and reduce stigma, ultimately ensuring better quality of life for affected individuals worldwide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021-2030 (WHO, 2021).
Röltgen, K., Johnson, P. D. R. & Pluschke, G. in Leprosy and Buruli Ulcer (eds Nunzi, E., Massone, C., Portaels, F.) 541–550 (Springer, 2022).
Yotsu, R. R. et al. Buruli ulcer: a review of the current knowledge. Curr. Trop. Med. Rep. 5, 247–256 (2018).
Omansen, T. F. et al. Global epidemiology of Buruli ulcer, 2010-2017, and analysis of 2014 WHO programmatic targets. Emerg. Infect. Dis. 25, 2183–2190 (2019).
Ravindran, B. et al. Epidemiology of Buruli ulcer in Victoria, Australia, 2017-2022. Emerg. Infect. Dis. 31, 448–457 (2025).
Fukaura, R. et al. Buruli ulcer: an epidemiological update from Japan. J. Dermatol. https://doi.org/10.1111/1346-8138.17483 (2025).
World Health Organization. Buruli ulcer https://www.who.int/data/gho/data/themes/topics/buruli-ulcer (WHO, 2024).
Kaser, M. et al. Evolution of two distinct phylogenetic lineages of the emerging human pathogen Mycobacterium ulcerans. BMC Evol. Biol. 7, 177 (2007).
Doig, K. D. et al. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genomics 13, 258 (2012).
Briand, M. et al. Emergence and spread of Mycobacterium ulcerans at different geographic scales. Microbiol. Spectr. 12, e0382723 (2024).
George, K. M. et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283, 854–857 (1999). Identified and characterized the biological effects of mycolactone, the key virulence factor of M. ulcerans.
Hall, B. S. et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 10, e1004061 (2014). A paradigm-shifting paper, by a process of elimination of molecular events in the biosynthesis of cytokines, identified for the first time mycolactone’s action at the ER, inhibiting the Sec61 translocon and thus opening up this new understanding of its pathogenic action.
Baron, L. et al. Mycolactone subverts immunity by selectively blocking the Sec61 translocon. J. Exp. Med. 213, 2885–2896 (2016).
Hong, H., Demangel, C., Pidot, S. J., Leadlay, P. F. & Stinear, T. Mycolactones: immunosuppressive and cytotoxic polyketides produced by aquatic mycobacteria. Nat. Prod. Rep. 25, 447–454 (2008).
Pidot, S. J. et al. Deciphering the genetic basis for polyketide variation among mycobacteria producing mycolactones. BMC Genomics 9, 462 (2008).
Tobias, N. J. et al. Complete genome sequence of the frog pathogen Mycobacterium ulcerans ecovar Liflandii. J. Bacteriol. 195, 556–564 (2013).
Combe, M., Cherif, E., Blaizot, R., Breugnot, D. & Gozlan, R. E. What about current diversity of mycolactone-producing mycobacteria? Implication for the diagnosis and treatment of Buruli ulcer. Int. J. Mol. Sci. 24, 13727 (2023).
Nienhuis, W. A. et al. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375, 664–672 (2010).
Yotsu, R. R., Richardson, M. & Ishii, N. Drugs for treating Buruli ulcer (Mycobacterium ulcerans disease). Cochrane Database Syst. Rev. 8, CD012118 (2018).
Phillips, R. O. et al. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet 395, 1259–1267 (2020). Demonstrated that all-oral 8-week treatment with a rifampicin–clarithromycin regimen is non-inferior to a rifampicin–streptomycin injectable regimen and is associated with fewer adverse events, establishing the current standard of care.
Converse, P. J., Almeida, D. V., Tyagi, S., Xu, J. & Nuermberger, E. L. Shortening Buruli ulcer treatment with combination therapy targeting the respiratory chain and exploiting Mycobacterium ulcerans gene decay. Antimicrob. Agents Chemother. 63, e00426-19 (2019).
Singh, S., Yotsu, R., N’uremberger, E. & Sirivastava, S. Repurposing drugs to advane the treatment of Buruli ulcer. Antimicrob. Agents Chemother. https://doi.org/10.1128/aac.00029-25 (2025).
Scherr, N. et al. Targeting the Mycobacterium ulcerans cytochrome bc1:aa3 for the treatment of Buruli ulcer. Nat. Commun. 9, 5370 (2018). Demonstrated that M. ulcerans is highly sensitive to telacebec because lineages from both Africa and Australia have no functional alternate terminal oxidases, making the drug a promising candidate for Buruli ulcer treatment.
World Health Organization. Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers (WHO, 2012).
Fyfe, J. A. et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 4, e791 (2010).
Vandelannoote, K. et al. Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. Elife 12, e84983 (2023).
Mee, P. T. et al. Mosquitoes provide a transmission route between possums and humans for Buruli ulcer in southeastern Australia. Nat. Microbiol. 9, 377–389 (2024).
Lavender, C. J. et al. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PLoS Negl. Trop. Dis. 5, e1305 (2011).
Buultjens, A. H. et al. Mosquitoes as vectors of Mycobacterium ulcerans based on analysis of notifications of alphavirus infection and Buruli ulcer, Victoria, Australia. Emerg. Infect. Dis. 30, 1918–1921 (2024).
Douine, M. et al. Mycobacterium ulcerans infection (Buruli ulcer) in French Guiana, South America, 1969-2013: an epidemiological study. Lancet Planet. Health 1, e65–e73 (2017).
McNamara, B. J. et al. Comprehensive case-control study of protective and risk factors for Buruli ulcer, Southeastern Australia. Emerg. Infect. Dis. 29, 2032–2043 (2023).
Boccarossa, A. et al. A combined field study of Buruli ulcer disease in southeast Benin proposing preventive strategies based on epidemiological, geographic, behavioural and environmental analyses. PLOS Glob. Public. Health 2, e0000095 (2022).
World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a strategic framework for integrated control and management of skin-related neglected tropical diseases (WHO, 2022).
Yotsu, R. R. et al. A global call for action to tackle skin-related neglected tropical diseases (skin NTDs) through integration: an ambitious step change. PLoS Negl. Trop. Dis. 17, e0011357 (2023).
Yotsu, R. R. et al. World Health Organization strategic framework for integrated control and management of skin-related neglected tropical diseases: what does this mean for dermatologists? Br. J. Dermatol. 188, 157–159 (2023).
World Health Organization Buruli ulcer disease: Mycobacterium ulcerans infection. Wkly Epidemiol. Rec. 77, 271–275 (2002).
Clancey, J. K. Mycobacterial skin ulcers in Uganda: description of a new mycobacterium (Mycobacterium buruli). J. Pathol. Bacteriol. 88, 175–187 (1964).
Nail, A. M. A., Tonga, R. A., Salih, H. M., Abuzeid, N. & Ahmed, M. H. The co-infection of Buruli ulcer and cutaneous leishmaniasis in Sudanese patient: an association by choice or by chance? J. Infect. Public. Health 13, 1184–1186 (2020).
Maccallum, P., Tolhurst, J. C., Buckle, G. & Sissons, H. A. A new mycobacterial infection in man. J. Pathol. Bacteriol. 60, 93–122 (1948).
Johnson, P. D. R. in Buruli Ulcer: Mycobacterium ulcerans Disease (eds Pluschke, G. & Röltgen, K.) 61–76 (Springer, 2019).
Buultjens, A. H. et al. Comparative genomics shows that Mycobacterium ulcerans migration and expansion preceded the rise of Buruli ulcer in Southeastern Australia. Appl Environ. Microbiol 84, e02612-17 (2018).
Johnson, P. D., Veitch, M. G., Leslie, D. E., Flood, P. E. & Hayman, J. A. The emergence of Mycobacterium ulcerans infection near Melbourne. Med. J. Aust. 164, 76–78 (1996).
Boyd, S. C. et al. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med. J. Aust. 196, 341–344 (2012).
Loftus, M. J. et al. Epidemiology of Buruli ulcer infections, Victoria, Australia, 2011-2016. Emerg. Infect. Dis. 24, 1988–1997 (2018).
Steffen, C. M. & Freeborn, H. Mycobacterium ulcerans in the Daintree 2009-2015 and the mini-epidemic of 2011. ANZ J. Surg. 88, E289–E293 (2018).
Hossain, M. E. et al. Buruli ulcer in Australia: evidence for a new endemic focus at Batemans Bay, New South Wales. PLoS Negl. Trop. Dis. 18, e0012702 (2024).
Lavender, C. J. et al. First case of Mycobacterium ulcerans disease (Bairnsdale or Buruli ulcer) acquired in New South Wales. Med. J. Aust. 186, 62–63 (2007).
Mikoshiba, H., Shindo, Y., Matsumoto, H., Mochizuki, M. & Tsukamura, M. A case of typical mycobacteriosis due to Mycobacterium ulcerans-like organism (author’s transl) [Japanese]. Nihon Hifuka Gakkai Zasshi 92, 557–565 (1982).
Yotsu, R. R., Nakanaga, K., Hoshino, Y., Suzuki, K. & Ishii, N. Buruli ulcer and current situation in Japan: a new emerging cutaneous mycobacterium infection. J. Dermatol. 39, 587–593 (2012).
Yotsu, R. R. et al. Revisiting Buruli ulcer. J. Dermatol. 42, 1033–1041 (2015).
Guerra Laso, J. M. et al. Buruli ulcers in a Spanish aid worker after a stay in Peru. Int. J. Infect. Dis. 78, 99–102 (2019).
Thomas, B. S. et al. Mycobacterium ulcerans infection imported from Australia to Missouri, USA, 2012. Emerg. Infect. Dis. 20, 1876–1879 (2014).
Bohelay, G. et al. Extensive buruli ulcer in a patient returning from Mali and Senegal. Travel. Med. Infect. Dis. 60, 102725 (2024).
Norman, F. F. & Chen, L. H. Imported Buruli ulcer — is there risk for travellers? J. Travel. Med. 31, taae057 (2024).
Faber, W. R. et al. First reported case of Mycobacterium ulcerans infection in a patient from China. Trans. R. Soc. Trop. Med. Hyg. 94, 277–279 (2000).
Vincent, Q. B. et al. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: a cohort study. Lancet Glob. Health 2, e422–e430 (2014).
N’Krumah, R. T. A. S. et al. Characteristics and epidemiological profile of Buruli ulcer in the district of Tiassale, south Cote d’Ivoire. Acta Tropica 175, 138–144 (2017).
Amoako, Y. A. et al. Clinical epidemiology of Buruli ulcer disease in Ghana, 2006-2024. Clin. Exp. Dermatol. https://doi.org/10.1093/ced/llaf326 (2025).
Röltgen, K. & Pluschke, G. in Buruli Ulcer: Mycobacterium ulcerans Disease (eds Pluschke, G. & Röltgen, K.) 1–41 (Springer, 2019).
Bratschi, M. W. et al. Geographic distribution, age pattern and sites of lesions in a cohort of Buruli ulcer patients from the Mapé Basin of Cameroon. PLoS Negl. Trop. Dis. 7, e2252 (2013).
Röltgen, K. et al. Late onset of the serological response against the 18 kDa small heat shock protein of Mycobacterium ulcerans in children. PLoS Negl. Trop. Dis. 8, e2904 (2014).
Röltgen, K., Pluschke, G., Spencer, J. S., Brennan, P. J. & Avanzi, C. The immunology of other mycobacteria: M. ulcerans, M. leprae. Semin. Immunopathol. 42, 333–353 (2020).
Johnson, H. et al. Territorial and gender-linked risk factors for Buruli ulcer in Southern Benin: a case-control study using geographic and behavioral surveying. PLoS Negl. Trop. Dis. 19, e0013509 (2025).
Kanga, J. M. et al. Recurrence after surgical treatment of Buruli ulcer in Cote d’Ivoire [French]. Bull. Soc. Pathol. Exot. 96, 406–409 (2003).
Amofah, G., Asamoah, S. & Afram-Gyening, C. Effectiveness of excision of pre-ulcerative Buruli lesions in field situations in a rural district in Ghana. Tropical Dr. 28, 81–83 (1998).
Kibadi, K., Mputu-Yamba, J. B., Mokassa, B., Panda, M. & Muyembe-Tamfum, J. J. Relapse after surgical treatment of Mycobacterium ulcerans infection (Buruli ulcer): study of risk factors in 84 patients in the Democratic Republic of the Congo [French]. Med. Trop. 69, 471–474 (2009).
Teelken, M. A. et al. Buruli ulcer: differences in treatment outcome between two centres in Ghana. Acta Trop. 88, 51–56 (2003).
Beissner, M. et al. Treatment outcome of patients with Buruli ulcer disease in Togo. PLoS Negl. Trop. Dis. 9, e0004170 (2015).
Addison, N. O. et al. Assessing and managing wounds of Buruli ulcer patients at the primary and secondary health care levels in Ghana. PLoS Negl. Trop. Dis. 11, e0005331 (2017).
Kpeli, G. et al. Possible healthcare-associated transmission as a cause of secondary infection and population structure of Staphylococcus aureus isolates from two wound treatment centres in Ghana. New Microbes New Infect. 13, 92–101 (2016).
Kpeli, G. S. & Yeboah-Manu, D. in Buruli Ulcer: Mycobacterium ulcerans Disease (eds Pluschke, G. & Röltgen, K.) 227–239 (Springer, 2019).
Ackam, N. et al. Antimicrobial resistant bacteria isolated from Buruli ulcer lesions in Ghana. PLoS Negl. Trop. Dis. 19, e0013140 (2025).
Phanzu, D. M. et al. Mycobacterium ulcerans disease (Buruli ulcer) in a rural hospital in Bas-Congo, Democratic Republic of Congo, 2002-2004. Am. J. Trop. Med. Hyg. 75, 311–314 (2006).
Coutts, S. P., Lau, C. L., Field, E. J., Loftus, M. J. & Tay, E. L. Delays in patient presentation and diagnosis for Buruli Ulcer (Mycobacterium ulcerans infection) in Victoria, Australia, 2011-2017. Trop. Med. Infect. Dis. 4, 100 (2019).
O’Brien, D. P., Friedman, N. D., Walton, A., Hughes, A. & Athan, E. Risk Factors associated with antibiotic treatment failure of Buruli ulcer. Antimicrob. Agents Chemother. 64, e00722-20 (2020).
Tchatchouang, S. et al. Systematic review: global host range, case fatality and detection rates of Mycobacterium ulcerans in humans and potential environmental sources. J. Clin. Tuberc. Other Mycobact. Dis. 36, 100457 (2024).
Bayonne Manou, L. S. et al. Mycobacterium ulcerans disease (Buruli ulcer) in Gabon: 2005-2011 [French]. Med. Sante Trop. 23, 450–457 (2013).
O’Brien, D. P. et al. Mycobacterium ulcerans in the elderly: more severe disease and suboptimal outcomes. PLoS Negl. Trop. Dis. 9, e0004253 (2015).
O’Brien, D. P., Christinet, V. & Ford, N. in Buruli Ulcer: Mycobacterium ulcerans Disease (eds Pluschke, G. & Röltgen, K.) 241–248 (Springer, 2019).
Christinet, V. et al. Impact of human immunodeficiency virus on the severity of Buruli ulcer disease: results of a retrospective study in Cameroon. Open Forum Infect. Dis. 1, ofu021 (2014).
Osei-Owusu, J. et al. Buruli ulcer in Africa: geographical distribution, ecology, risk factors, diagnosis, and indigenous plant treatment options — a comprehensive review. Heliyon 9, e22018 (2023).
O’Brien, D. P., Jeanne, I., Blasdell, K., Avumegah, M. & Athan, E. The changing epidemiology worldwide of Mycobacterium ulcerans. Epidemiol. Infect. 147, e19 (2018).
van der Werf, T. S., van der Graaf, W. T., Groothuis, D. G. & Knell, A. J. Mycobacterium ulcerans infection in Ashanti region, Ghana. Trans. R. Soc. Trop. Med. Hyg. 83, 410–413 (1989).
Ohtsuka, M. et al. Buruli ulcer caused by Mycobacterium ulcerans subsp shinshuense: a rare case of familial concurrent occurrence and detection of insertion sequence 2404 in Japan. JAMA Dermatol. 150, 64–67 (2014).
McNamara, B. J. et al. Mycobacterium ulcerans in possum feces before emergence in humans, Australia. Emerg. Infect. Dis. 31, 569–573 (2025).
Johnson, P. D. et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg. Infect. Dis. 13, 1653–1660 (2007).
Yerramilli, A. et al. The location of Australian Buruli ulcer lesions — implications for unravelling disease transmission. PLoS Negl. Trop. Dis. 11, e0005800 (2017).
Quek, T. Y. et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg. Infect. Dis. 13, 1661–1666 (2007).
Röltgen, K., Pluschke, G., Johnson, P. D. R. & Fyfe, J. Mycobacterium ulcerans DNA in bandicoot excreta in Buruli ulcer-endemic area, Northern Queensland, Australia. Emerg. Infect. Dis. 23, 2042–2045 (2017).
Singh, A., McBride, W. J. H., Govan, B., Pearson, M. & Ritchie, S. A. A survey on Mycobacterium ulcerans in Mosquitoes and March flies captured from endemic areas of Northern Queensland, Australia. PLoS Negl. Trop. Dis. 13, e0006745 (2019).
Garchitorena, A. et al. Mycobacterium ulcerans dynamics in aquatic ecosystems are driven by a complex interplay of abiotic and biotic factors. Elife 4, e07616 (2015).
Merritt, R. W. et al. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl. Trop. Dis. 4, e911 (2010).
Marsollier, L. et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 3, e62 (2007).
Marsollier, L. et al. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl. Env. Microbiol. 70, 1097–1103 (2004).
Durnez, L. et al. Terrestrial small mammals as reservoirs of Mycobacterium ulcerans in benin. Appl. Env. Microbiol. 76, 4574–4577 (2010).
Williamson, H. R. et al. Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: implications for transmission. PLoS Negl. Trop. Dis. 8, e2770 (2014).
Ruf, M. T. et al. Spatial distribution of Mycobacterium ulcerans in Buruli ulcer lesions: implications for laboratory diagnosis. PLoS Negl. Trop. Dis. 10, e0004767 (2016).
Muhi, S. et al. A refined low-dose murine model of Mycobacterium ulcerans infection to assess integrated immune networks in Buruli ulcer pathogenesis. mBio 16, e0193125 (2025).
Loftus, M. J. et al. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection) in Victoria, Australia - Remains similar despite changing geographic distribution of disease. PLoS Negl. Trop. Dis. 12, e0006323 (2018).
Sexton-Oates, N. K., Stewardson, A. J., Yerramilli, A. & Johnson, P. D. R. Does skin surface temperature variation account for Buruli ulcer lesion distribution? PLoS Negl. Trop. Dis. 14, e0007732 (2020).
Yeboah-Manu, D. et al. Sero-epidemiology as a tool to screen populations for exposure to Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 6, e1460 (2012).
Röltgen, K. & Pluschke, G. Buruli ulcer: the efficacy of innate immune defense may be a key determinant for the outcome of infection with Mycobacterium ulcerans. Front. Microbiol. 11, 1018 (2020).
Silva, M. T., Portaels, F. & Pedrosa, J. Pathogenetic mechanisms of the intracellular parasite Mycobacterium ulcerans leading to Buruli ulcer. Lancet Infect. Dis. 9, 699–710 (2009).
Ruf, M. T., Steffen, C., Bolz, M., Schmid, P. & Pluschke, G. Infiltrating leukocytes surround early Buruli ulcer lesions, but are unable to reach the mycolactone producing mycobacteria. Virulence 8, 1918–1926 (2017).
Demangel, C. Immunity against Mycobacterium ulcerans: the subversive role of mycolactone. Immunol. Rev. 301, 209–221 (2021).
Bieri, R. et al. The macrolide toxin mycolactone promotes bim-dependent apoptosis in Buruli ulcer through inhibition of mTOR. ACS Chem. Biol. 12, 1297–1307 (2017).
Hsieh, L. T. et al. The Mycobacterium ulcerans toxin mycolactone causes destructive Sec61-dependent loss of the endothelial glycocalyx and vessel basement membrane to drive skin necrosis. Elife 12, e86931 (2025).
Demangel, C. & High, S. Sec61 blockade by mycolactone: a central mechanism in Buruli ulcer disease. Biol. Cell 110, 237–248 (2018).
McKenna, M., Simmonds, R. E. & High, S. Mechanistic insights into the inhibition of Sec61-dependent co- and post-translational translocation by mycolactone. J. Cell Sci. 129, 1404–1415 (2016).
Hayman, J. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46, 5–9 (1993).
Hong, H. et al. Mycolactone diffuses from Mycobacterium ulcerans-infected tissues and targets mononuclear cells in peripheral blood and lymphoid organs. PLoS Negl. Trop. Dis. 2, e325 (2008).
O’Keefe, S., Pool, M. R. & High, S. Membrane protein biogenesis at the ER: the highways and byways. FEBS J. 289, 6835–6862 (2022).
Gérard, S. F. et al. Structure of the inhibited state of the Sec translocon. Mol. Cell 79, 406–415.e7 (2020).
Zong, G. et al. Ipomoeassin F binds Sec61α to inhibit protein translocation. J. Am. Chem. Soc. 141, 8450–8461 (2019).
Lang, S. et al. Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J. Cell Sci. 125, 1958–1969 (2012).
Morel, J. D. et al. Proteomics reveals scope of mycolactone-mediated Sec61 blockade and distinctive stress signature. Mol. Cell Proteom. 17, 1750–1765 (2018).
Ogbechi, J. et al. Inhibition of Sec61-dependent translocation by mycolactone uncouples the integrated stress response from ER stress, driving cytotoxicity via translational activation of ATF4. Cell Death Dis. 9, 397 (2018).
Isaac, C. et al. Mycolactone displays anti-inflammatory effects on the nervous system. PLoS Negl. Trop. Dis. 11, e0006058 (2017).
Grotzke, J. E. et al. Sec61 blockade by mycolactone inhibits antigen cross-presentation independently of endosome-to-cytosol export. Proc. Natl Acad. Sci. USA 114, E5910–E5919 (2017).
Foulon, M. et al. Mycolactone toxin induces an inflammatory response by targeting the IL-1β pathway: mechanistic insight into Buruli ulcer pathophysiology. PLoS Pathog. 16, e1009107 (2020).
Hall, B. S., Hsieh, L. T., Sacre, S. & Simmonds, R. E. The one that got away: how macrophage-derived IL-1β escapes the mycolactone-dependent Sec61 blockade in Buruli ulcer. Front. Immunol. 12, 788146 (2022).
Schutte, D. & Pluschke, G. Immunosuppression and treatment-associated inflammatory response in patients with Mycobacterium ulcerans infection (Buruli ulcer). Expert. Opin. Biol. Ther. 9, 187–200 (2009).
En, J. et al. Mycolactone cytotoxicity in Schwann cells could explain nerve damage in Buruli ulcer. PLoS Negl. Trop. Dis. 11, e0005834 (2017).
Goto, M. et al. Nerve damage in Mycobacterium ulcerans-infected mice: probable cause of painlessness in Buruli ulcer. Am. J. Pathol. 168, 805–811 (2006).
Marion, E. et al. Mycobacterial toxin induces analgesia in Buruli ulcer by targeting the angiotensin pathways. Cell 157, 1565–1576 (2014).
Junghanss, T. & Pluschke, G. in Manson’s Tropical Diseases 24th edn Ch. 48 (eds Farrar, J. et al.) 557–568 (Elsevier, 2024).
Martins, T. G. et al. Local and regional re-establishment of cellular immunity during curative antibiotherapy of murine Mycobacterium ulcerans infection. PLoS ONE 7, e32740 (2012).
Schutte, D. et al. Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin. PLoS Negl. Trop. Dis. 1, e2 (2007).
Hsieh, L. T. et al. Aberrant stromal tissue factor localisation and mycolactone-driven vascular dysfunction, exacerbated by IL-1β, are linked to fibrin formation in Buruli ulcer lesions. PLoS Pathog. 18, e1010280 (2022).
Ogbechi, J. et al. Mycolactone-dependent depletion of endothelial cell thrombomodulin is strongly associated with fibrin deposition in Buruli ulcer lesions. PLoS Pathog. 11, e1005011 (2015).
World Health Organization. Updated Buruli Ulcer Recording and Reporting Forms Are Now Available https://www.who.int/news/item/24-08-2020-updated-buruli-ulcer-recording-and-reporting-forms-are-now-available (WHO, 2020).
Owusu, E., Newman, M. J., Akumwena, A., Pfosu-Appiah, L. & Pluschke, G. Maximizing microscopy as a diagnostic tool in peripheral health centres of BU endemic areas in Ghana. Int. J. Mycobacteriology 4, 184–190 (2015).
Röltgen, K., Cruz, I., Ndung’u, J. M. & Pluschke, G. in Buruli Ulcer: Mycobacterium ulcerans Disease (eds Pluschke, G. & Röltgen, K.) 183–202 (Springer, 2019).
Sakyi, S. A., Aboagye, S. Y., Darko Otchere, I. & Yeboah-Manu, D. Clinical and laboratory diagnosis of Buruli ulcer disease: a systematic review. Can. J. Infect. Dis. Med. Microbiol. 2016, 5310718 (2016).
Portaels, F. Laboratory Diagnosis of Buruli Ulcer: A Manual for Health Care Providers (WHO, 2014).
Singh, S., Yotsu, R., Nuremberger, E. & Srivastava, S. Repurposing drugs to advance the treatment of Buruli ulcer. Antimicrob. Agents Chemother. 69, e0002925 (2025).
Guarner, J. et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 9, 651–656 (2003).
Ross, B. C. et al. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35, 1696–1700 (1997). Identified insertion sequences in the genome of M. ulcerans that serve as targets for the PCR diagnostic assay for Buruli ulcer.
Stinear, T. et al. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J. Clin. Microbiol. 37, 1018–1023 (1999).
Stienstra, Y. van, der Werf, T. S. et al. Analysis of an IS2404-based nested PCR for diagnosis of Buruli ulcer disease in regions of Ghana where the disease is endemic. J. Clin. Microbiol. 41, 794–797 (2003).
Fyfe, J. A. et al. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl. Env. Microbiol. 73, 4733–4740 (2007).
Beissner, M. et al. Development of a combined RLEP/16S rRNA (RT) qPCR assay for the detection of viable M. leprae from nasal swab samples. BMC Infect. Dis. 19, 753 (2019).
Bretzel, G. et al. External quality assurance for the laboratory diagnosis of Buruli ulcer disease in Ghana. Trop. Med. Int. Health 11, 1688–1693 (2006).
Eddyani, M. et al. Multicenter external quality assessment program for PCR detection of Mycobacterium ulcerans in clinical and environmental specimens. PLoS ONE 9, e89407 (2014).
Marion, E. et al. A combined effort of 11 laboratories in the WHO African region to improve quality of Buruli ulcer PCR diagnosis: The “BU-LABNET. PLoS Negl. Trop. Dis. 16, e0010908 (2022).
World Health Organization. Transitioning from BU-LABNET to Skin NTD LABNET https://www.who.int/news/item/03-11-2023-transitioning-from-bu-labnet-to-skin-ntd-labnet (WHO, 2023).
Frimpong, M. et al. Multi-centric evaluation of Biomeme Franklin Mobile qPCR for rapid detection of Mycobacterium ulcerans in clinical specimens. PLoS Negl. Trop. Dis. 17, e0011373 (2023).
de Souza, D. K., Quaye, C., Mosi, L., Addo, P. & Boakye, D. A. A quick and cost effective method for the diagnosis of Mycobacterium ulcerans infection. BMC Infect. Dis. 12, 8 (2012).
Manjunatha, C. et al. Isothermal amplification techniques: an emerging tool for on-site detection of phytopathogens in field conditions. Methods Mol. Biol. 2943, 47–64 (2025).
Babonneau, J. et al. Development of a dry-reagent-based qPCR to facilitate the diagnosis of Mycobacterium ulcerans infection in endemic countries. PLoS Negl. Trop. Dis. 9, e0003606 (2015).
Boakye-Appiah, J., Hall, B., Reljic, R. & Simmonds, R. E. in Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges (ed. Christodoulides, M.) 71–95 (Springer, 2023).
Muhi, S. & Stinear, T. P. Systematic review of M. bovis BCG and other candidate vaccines for Buruli ulcer prophylaxis. Vaccine 39, 7238–7252 (2021).
Smith, P. G., Revill, W. D., Lukwago, E. & Rykushin, Y. P. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans. R. Soc. Trop. Med. Hyg. 70, 449–457 (1976).
Forrest, G. N. & Tamura, K. Rifampin combination therapy for nonmycobacterial infections. Clin. Microbiol. Rev. 23, 14–34 (2010).
Muhi, S. et al. Management of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: consensus statement. Med. J. Aust. 222, 571–578 (2025).
Sugawara, M. et al. Exploration of a standard treatment for Buruli ulcer through a comprehensive analysis of all cases diagnosed in Japan. J. Dermatol. 42, 588–595 (2015).
Amoako, Y. et al. Buruli-RifDACC: evaluation of the efficacy and cost-effectiveness of high-dose versus standard-dose rifampicin on outcomes in Mycobacterium ulcerans disease, a protocol for a randomised controlled trial in Ghana. NIHR Open. Res. 2, 59 (2022).
Johnson, R. C. et al. Comparison of 8 weeks standard treatment (rifampicin plus clarithromycin) vs. 4 weeks standard plus amoxicillin/clavulanate treatment [RC8 vs. RCA4] to shorten Buruli ulcer disease therapy (the BLMs4BU trial): study protocol for a randomized controlled multi-centre trial in Benin. Trials 23, 559 (2022).
Zumla, A., Nahid, P. & Cole, S. T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug. Discov. 12, 388–404 (2013).
Sotgiu, G. et al. Applicability of the shorter ‘Bangladesh regimen’ in high multidrug-resistant tuberculosis settings. Int. J. Infect. Dis. 56, 190–193 (2017).
World Health Organization. The Shorter MDR-TB Regimen https://www.aidsdatahub.org/sites/default/files/resource/shorter-treatment-regimens-multidrug-resistant-tuberculosis-mdr-tb.pdf (WHO, 2016).
Moodley, R., Godec, T. R. & Team, S. T. Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur. Respir. Rev. 25, 29–35 (2016).
Srivastava, S. et al. Nouveau short-course therapy and morphism mapping for clinical pulmonary Mycobacterium kansasii. Antimicrob. Agents Chemother. 95, e01553-20 (2023).
Dorman, S. E. et al. High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial. Contemp. Clin. Trials 90, 105938 (2020).
Nunn, A. J. et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 380, 1201–1213 (2019).
Srivastava, S., Deshpande, D., Magombedze, G. & Gumbo, T. Efficacy versus hepatotoxicity of high-dose rifampin, pyrazinamide, and moxifloxacin to shorten tuberculosis therapy duration: there is still fight in the old warriors yet!. Clin. Infect. Dis. 67, S359–S364 (2018).
Singh, S. et al. Imipenem pharmacokinetics/pharmacodynamics in preclinical hollow fiber model, dose finding in virtual patients, and clinical evidence of efficacy for Mycobacterium abscessus lung disease. J Infect. Dis. 231, 1521–1531 (2025).
Singh, S. et al. Omadacycline pharmacokinetics/pharmacodynamics in the hollow fiber model and clinical validation of efficacy to treat pulmonary Mycobacterium abscessus disease. Int. J. Antimicrob. Agents 62, 106847 (2023).
Siddiqa, A. et al. Omadacycline for the treatment of Mycobacterium abscessus infections: case series and review of the literature. IDCases 31, e01703 (2023).
Deshpande, D. et al. Ceftriaxone efficacy for Mycobacterium avium complex lung disease in the hollow fiber and translation to sustained sputum culture conversion in patients. J. Infect. Dis. https://doi.org/10.1093/infdis/jiad545 (2023).
Deshpande, D., Srivastava, S., Pasipanodya, J. G. & Gumbo, T. Minocycline intra-bacterial pharmacokinetic hysteresis as a basis for pharmacologic memory and a backbone for once-a-week pan-tuberculosis therapy. Front. Pharmacol. 13, 1024608 (2022).
Deshpande, D., Srivastava, S., Pasipanodya, J. G., Lee, P. S. & Gumbo, T. A novel ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) regimen for pulmonary Mycobacterium avium disease. J. Antimicrob. Chemother. 72, i48–i53 (2017).
Almeida, D. V. et al. Telacebec for ultrashort treatment of Buruli ulcer in a mouse model. Antimicrob. Agents Chemother. 64, e00259-20 (2020).
Converse, P. J., Nuermberger, E. L., Almeida, D. V. & Grosset, J. H. Treating Mycobacterium ulcerans disease (Buruli ulcer): from surgery to antibiotics, is the pill mightier than the knife? Future Microbiol. 6, 1185–1198 (2011).
Warryn, L. & Pluschke, G. Repurposing of tuberculosis drug candidates for the treatment of Mycobacterium ulcerans disease. Chimia 77, 577–581 (2023).
Zhang, T., Bishai, W. R., Grosset, J. H. & Nuermberger, E. L. Rapid assessment of antibacterial activity against Mycobacterium ulcerans by using recombinant luminescent strains. Antimicrob. Agents Chemother. 54, 2806–2813 (2010).
Zhang, T. et al. Using bioluminescence to monitor treatment response in real time in mice with Mycobacterium ulcerans infection. Antimicrob. Agents Chemother. 55, 56–61 (2011).
Zhang, T., Li, S. Y., Converse, P. J., Grosset, J. H. & Nuermberger, E. L. Rapid, serial, non-invasive assessment of drug efficacy in mice with autoluminescent Mycobacterium ulcerans infection. PLoS. Negl. Trop. Dis. 7, e2598 (2013).
Boorgula, G. D. et al. Omadacycline drug susceptibility testing for non-tuberculous mycobacteria using oxyrase to overcome challenges with drug degradation. Tuberculosis 147, 102519 (2024).
Stinear, T. P. et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 18, 729–741 (2008).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06481163 (2025).
Velding, K. et al. Wound care in Buruli ulcer disease in Ghana and Benin. Am. J. Trop. Med. Hyg. 91, 313–318 (2014).
Ugai, K., Koffi, D. Y., Kouadio, K., Yao, A. & Yotsu, R. R. Nutritional status and wound healing in patients with Mycobacterium ulcerans disease (Buruli ulcer): a pilot study from rural Cote d’Ivoire. Eur. J. Dermatol. 32, 227–236 (2022).
Koffi, D. Y. et al. Accelerating the healing of hard-to-heal wounds with food supplements: nutritional analysis in the Cote d’Ivoire. J. Wound Care 32, cci–ccx (2023).
Koffi, Y. D., Koffi, P. A., Ehouman, E., Kouadio, S. & Kaloga, M. Efficacy of nutritional support in combination with standard Buruli ulcer treatment: a case study in Côte d’Ivoire. J. Clin. Tuberculosis Other Mycobact. Dis. 37, 100496 (2024).
Loglo, A. et al. Micronutrient-deficient diets and possible environmental enteric dysfunction in Buruli ulcer endemic communities in Ghana: lower dietary diversity and reduced serum zinc and vitamin C implicate micronutrient status a possible susceptibility factor. PLoS Negl. Trop. Dis. 19, e0012871 (2025).
O’Brien, D. P. et al. Mycobacterium ulcerans disease management in Australian patients: the re-emergence of surgery as an important treatment modality. ANZ J. Surg. 89, 653–658 (2019).
Degboe, B. et al. Buruli ulcer: evaluation of its medical and surgical management at the Allada (Benin) screening and treatment center, 2010-2014. Med. Sante Trop. 29, 402–408 (2019).
Lim, B. et al. Mycobacterium Ulcerans Ulcer: current trends in antimicrobial management and reconstructive surgical strategies. Life 15, 1096 (2025).
Tuffour, J. et al. Challenges associated with management of Buruli ulcer/human immunodeficiency virus coinfection in a treatment center in Ghana: a case series study. Am. J. Trop. Med. Hyg. 93, 216–223 (2015).
Frimpong, M. et al. Paradoxical reactions in Buruli ulcer after initiation of antibiotic therapy: relationship to bacterial load. PLoS Negl. Trop. Dis. 13, e0007689 (2019).
Sarpong-Duah, M. et al. Clearance of viable Mycobacterium ulcerans from Buruli ulcer lesions during antibiotic treatment as determined by combined 16S rRNA reverse transcriptase /IS 2404 qPCR assay. PLoS Negl. Trop. Dis. 11, e0005695 (2017).
Agbavor, B. et al. Clinical and microbiological predictors of healing in Buruli ulcer disease. J. Clin. Tuberculosis Other Mycobact. Dis. 34, 100415 (2024).
O’Brien, D. P. et al. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med. J. Aust. 200, 267–270 (2014).
Nienhuis, W. A. et al. Paradoxical responses after start of antimicrobial treatment in Mycobacterium ulcerans infection. Clin. Infect. Dis. 54, 519–526 (2012).
Klis, S., Kingma, R., Tuah, W., Stienstra, Y. & van der Werf, T. S. Compliance with antimicrobial therapy for buruli ulcer. Antimicrob. Agents Chemother. 58, 6340 (2014).
Phillips, R. O. et al. Reply to “Compliance with antimicrobial therapy for buruli ulcer. Antimicrob. Agents Chemother. 58, 6341 (2014).
Collinson, S. et al. Barriers to Buruli ulcer treatment completion in the Ashanti and Central Regions, Ghana. PLoS Negl. Trop. Dis. 14, e0008369 (2020).
Alferink, M. et al. Perceptions on the effectiveness of treatment and the timeline of Buruli ulcer influence pre-hospital delay reported by healthy individuals. PLoS Negl. Trop. Dis. 7, e2014 (2013).
Butler, J. et al. Multisensory medical illustrations of Buruli ulcer for improved disease detection, help seeking behaviour and adherence to treatment. J. Vis. Commun. Med. 47, 8–20 (2024).
Klis, S., Kingma, R. A., Tuah, W., van der Werf, T. S. & Stienstra, Y. Clinical outcomes of Ghanaian Buruli ulcer patients who defaulted from antimicrobial therapy. Trop. Med. Int. Health 21, 1191–1196 (2016).
Yeboah-Manu, D. et al. Secondary bacterial infections of Buruli ulcer lesions before and after chemotherapy with streptomycin and rifampicin. PLoS Negl. Trop. Dis. 7, e2191 (2013).
Amoako, Y. A. et al. Mental health and quality of life burden in Buruli ulcer disease patients in Ghana. Infect. Dis. Poverty 10, 109 (2021).
Hailemichael, Y. et al. The role of economic factors in shaping and constituting the household burden of neglected tropical diseases of the skin: qualitative findings from Ghana and Ethiopia. Soc. Sci. Med. 356, 117094 (2024).
Hamzat, T. K. & Boakye-Afram, B. Health-related quality of life among persons living with Buruli ulcer in Amasaman Community, Ga West District Accra, Ghana. Int. J. Health Sci. 5, 29–38 (2011).
Grietens, K. P. et al. “It is me who endures but my family that suffers”: social isolation as a consequence of the household cost burden of Buruli ulcer free of charge hospital treatment. PLoS Negl. Trop. Dis. 2, e321 (2008).
Asiedu, K. & Etuaful, S. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59, 1015–1022 (1998).
Klis, S. et al. Good quality of life in former Buruli ulcer patients with small lesions: long-term follow-up of the BURULICO trial. PLoS Negl. Trop. Dis. 8, e2964 (2014).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Harding, T. W. et al. Mental disorders in primary health care: a study of their frequency and diagnosis in four developing countries. Psychol. Med. 10, 231–241 (1980).
[No authors listed] Development of the world health organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol. Med. 28, 551–558 (1998).
World Health Organization. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0) (WHO, 2010).
Stienstra, Y. et al. Reliability and validity of the Buruli ulcer functional limitation score questionnaire. Am. J. Trop. Med. Hyg. 72, 449–452 (2005).
de Zeeuw, J. et al. Persisting social participation restrictions among former Buruli ulcer patients in Ghana and Benin. PLoS Negl. Trop. Dis. 8, e3303 (2014).
Alosaimi, F. D. et al. Associations of foot ulceration with quality of life and psychosocial determinants among patients with diabetes; a case-control study. J. Foot Ankle Res. 12, 57 (2019).
Obindo, J. et al. Prevalence of depression and associated clinical and socio-demographic factors in people living with lymphatic filariasis in Plateau State, Nigeria. PLoS Negl. Trop. Dis. 11, e0005567 (2017).
Govindharaj, P., Srinivasan, S. & Darlong, J. Quality of life of persons affected by leprosy in an endemic district, West Bengal, India. Indian J. Dermatol. 63, 459–464 (2018).
Tuwor, R. D. et al. Stigma experiences, effects and coping among individuals affected by Buruli ulcer and yaws in Ghana. PLoS Negl. Trop. Dis. 18, e0012093 (2024).
Okyere, D. et al. Improving experiences of neglected tropical diseases of the skin: mixed methods formative research for development of a complex intervention in Atwima Mponua District, Ghana. PLoS Glob. Public. Health 4, e0002833 (2024).
Awah, P. K. et al. Developing a Buruli ulcer community of practice in Bankim, Cameroon: a model for Buruli ulcer outreach in Africa. PLoS Negl. Trop. Dis. 12, e0006238 (2018).
Ake, J. et al. People-centered strategies to mobilize people living with disabilities due to Neglected Tropical Diseases (PD-NTDs) to influence policy and programs: a mixed-methods study in Cote d’Ivoire. PLoS Negl. Trop. Dis. 19, e0013485 (2025).
Adjorlolo, S. et al. Readiness assessment of healthcare professionals to integrate mental health services into primary healthcare of persons with skin-neglected tropical diseases in Ghana: a structural equation modeling. Int. J. Env. Res. Public. Health 22, 991 (2025).
Adjorlolo, S. et al. Psychological distress, perceived stress, and public stigma in skin neglected tropical diseases in Ghana: a structural equation model of a cross-sectional survey. PLoS Negl. Trop. Dis. 19, e0013387 (2025).
Adekeye, O. et al. Exploring the well-being of people affected by skin NTDs in Kaduna and Kwara States, Nigeria: a photovoice and scoping review study. Int. Health 15, i100–i109 (2023).
Seekles, M. L. et al. Mental health, stigma and the quality of life of people affected by neglected tropical diseases of the skin in Kasai Province, Democratic Republic of the Congo: a sex-disaggregated analysis. Int. Health 15, iii28–iii36 (2023).
Diaz, D. et al. Use of the immunodominant 18-kiloDalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clin. Vaccin. Immunol. 13, 1314–1321 (2006).
Sakakibara, Y. et al. Pilot use of a mycolactone-specific lateral flow assay for Buruli ulcer: a case report from Japan. J. Clin. Tuberc. Other Mycobact. Dis. 36, 100469 (2024).
Amewu, R. K. et al. Evaluation of the fluorescent-thin layer chromatography (f-TLC) for the diagnosis of Buruli ulcer disease in Ghana. PLoS ONE 17, e0270235 (2022).
Pluschke, G. & Warryn, L. How our molecular understanding of the pathogenesis of Mycobacterium ulcerans infection can improve diagnosis of Buruli ulcer. Expert. Rev. Mol. Diagn. 24, 1–4 (2024).
Nausch, N. et al. Analysis of Mycobacterium ulcerans-specific T-cell cytokines for diagnosis of Buruli ulcer disease and as potential indicator for disease progression. PLoS Negl. Trop. Dis. 11, e0005415 (2017).
Loglo, A. D. et al. IFN-γ and IL-5 whole blood response directed against mycolactone polyketide synthase domains in patients with Mycobacterium ulcerans infection. PeerJ 6, e5294 (2018).
Pidot, S. J. et al. Serological evaluation of Mycobacterium ulcerans antigens identified by comparative genomics. PLoS Negl. Trop. Dis. 4, e872 (2010).
Niang, F. et al. Metabolomic profiles delineate mycolactone signature in Buruli ulcer disease. Sci. Rep. 5, 17693 (2015).
Dangy, J. P. et al. Antibody-mediated neutralization of the exotoxin mycolactone, the main virulence factor produced by Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 10, e0004808 (2016).
Naranjo, L. et al. Recombinant antibodies against mycolactone. Toxins 11, 346 (2019).
Warryn, L. et al. An antigen capture assay for the detection of mycolactone, the polyketide toxin of Mycobacterium ulcerans. J. Immunol. 206, 2753–2762 (2021).
Warryn, L. et al. Development of an ELISA for the quantification of mycolactone, the cytotoxic macrolide toxin of Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 14, e0008357 (2020).
Siirin, M. et al. Towards a Buruli ulcer rapid diagnostic test that targets mycolactone. Preprint at medRxiv https://doi.org/10.1101/2024.01.23.24301643 (2024).
Leigheb, G. et al. Ultrasonography for the monitoring of subcutaneous damage in Mycobacterium ulcerans infection (Buruli ulcer). Ultrasound Med. Biol. 34, 1554–1563 (2008).
Yotsu, R. R. et al. Buruli ulcer: application of thermography for remote diagnosis of a neglected tropical disease. Br. J. Dermatol. 189, 236–238 (2023).
Cano, M. et al. Evaluating the World Health Organization’s SkinNTDs app as a training tool for skin neglected tropical diseases in Ghana and Kenya: cross-sectional study. J. Med. Internet Res. 26, e51628 (2024).
Quilter, E. E. V., Butlin, C. R., Carrion, C. & Postigo, J. The WHO Skin NTD mobile application — a paradigm shift in leprosy diagnosis through artificial intelligence? Lepr. Rev. 95, e2024030 (2024).
Yotsu, R. R. et al. An mHealth app (eSkinHealth) for detecting and managing skin diseases in resource-limited settings: mixed methods pilot study. JMIR Dermatol. 6, e46295 (2023).
Yotsu, R. R., Ding, Z., Hamm, J. & Blanton, R. E. Deep learning for AI-based diagnosis of skin-related neglected tropical diseases: a pilot study. PLoS Negl. Trop. Dis. 17, e0011230 (2023).
Wang, J. et al. eSkinHealth: a multimodal dataset for neglected tropical skin diseases. Preprint at https://doi.org/10.48550/arXiv.2508.18608 (2025).
Yotsu, R. R. et al. Harnessing artificial intelligence for skin-related neglected tropical diseases (skin NTDs): opportunities, challenges and future directions. Int. Health 17, 792–794 (2025).
Impact Global Health. Smart Decisions: The G-FINDER 2024 Neglected Disease R&D Report (Impact Global Health, 2025).
Velding, K., Klis, S. A., Abass, K. M., van der Werf, T. S. & Stienstra, Y. The application of modern dressings to Buruli ulcers: results from a pilot implementation project in Ghana. Am. J. Trop. Med. Hyg. 95, 60–62 (2016).
Murase, C., Kono, M., Nakanaga, K., Ishii, N. & Akiyama, M. Buruli ulcer successfully treated with negative-pressure wound therapy. JAMA Dermatol. 151, 1137–1139 (2015).
Velink, A. et al. Former Buruli ulcer patients’ experiences and wishes may serve as a guide to further improve Buruli ulcer management. PLoS Negl. Trop. Dis. 10, e0005261 (2016).
World Health Organization. Proc. Seventy-Eighth World Health Assembly https://apps.who.int/gb/ebwha/pdf_files/WHA78/A78_R15-en.pdf (WHO, 2025).
Kolimi, P., Narala, S., Nyavanandi, D., Youssef, A. A. A. & Dudhipala, N. Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements. Cells 11, 2439 (2022).
Dabas, M., Schwartz, D., Beeckman, D. & Gefen, A. Application of artificial intelligence methodologies to chronic wound care and management: a scoping review. Adv. Wound Care 12, 205–240 (2023).
Kivity, S. et al. Optimising wound monitoring: can digital tools improve healing outcomes and clinic efficiency. J. Clin. Nurs. 33, 4014–4023 (2024).
World Health Organization. The Yamoussoukro Declaration on Buruli Ulcer https://www.who.int/publications/m/item/the-yamoussoukro-declaration-on-buruli-ulcer (WHO, 1998).
Stinear, T. P. et al. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl Acad. Sci. USA 101, 1345–1349 (2004).
Stinear, T. P. et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 17, 192–200 (2007). Described the complete genome sequence of M. ulcerans and a virulence plasmid required for production of mycolactone.
Dega, H., Robert, J., Bonnafous, P., Jarlier, V. & Grosset, J. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 44, 2367–2372 (2000).
Etuaful, S. et al. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob. Agents Chemother. 49, 3182–3186 (2005).
World Health Organization. Provisional Guidance on the Role of Specific Antibiotics in the Management of Mycobacterium Ulcerans Disease (Buruli Ulcer) https://www.who.int/publications/i/item/WHO-CDS-CPE-GBUI-2004.10 (WHO, 2004).
Phillips, R. O. et al. Clinical and bacteriological efficacy of rifampin-streptomycin combination for two weeks followed by rifampin and clarithromycin for six weeks for treatment of Mycobacterium ulcerans disease. Antimicrob. Agents Chemother. 58, 1161–1166 (2014).
World Health Organization. Report from the Meeting of the Buruli ulcer Technical Advisory Group. World Health Organization, Headquarters, Geneva, Switzerland, 21 March 2017 https://cdn.who.int/media/docs/default-source/ntds/buruli-ulcer/meeting-reports-who-tag-bu/2017-who-bu-tag-report.pdf?sfvrsn=56e331ae_8 (WHO, 2017).
Scherr, N. et al. Structure-activity relationship studies on the macrolide exotoxin mycolactone of Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 7, e2143 (2013).
McKenna, M., Simmonds, R. E. & High, S. Mycolactone reveals the substrate-driven complexity of Sec61-dependent transmembrane protein biogenesis. J. Cell Sci. 130, 1307–1320 (2017).
Hall, B. S. et al. Inhibition of the SEC61 translocon by mycolactone induces a protective autophagic response controlled by EIF2S1-dependent translation that does not require ULK1 activity. Autophagy 18, 841–859 (2022).
Ruf, M.-T. et al. Histopathological changes and clinical responses of Buruli ulcer plaque lesions during chemotherapy: a role for surgical removal of necrotic tissue? PLoS Negl. Trop. Dis. 5, e1334 (2011).
Sakakibara, Y. et al. A case of pediatric Buruli ulcer presenting with cellulitis-like symptoms [Japanese]. J. Jpn. Pediatr. Soc. 128, 1437–1442 (2024).
Kawashima, A. et al. Genome-wide screening identified SEC61A1 as an essential factor for mycolactone-dependent apoptosis in human premonocytic THP-1 cells. PLoS Negl. Trop. Dis. 16, e0010672 (2022).
Robeson, L. et al. Characterization of the interaction between the Sec61 translocon complex and ppαF using optical tweezers. Protein Sci. 33, e4996 (2024).
Itskanov, S. et al. A common mechanism of Sec61 translocon inhibition by small molecules. Nat. Chem. Biol. https://doi.org/10.1038/s41589-023-01337-y (2023).
Bhadra, P. et al. Mycolactone enhances the Ca2+ leak from endoplasmic reticulum by trapping Sec61 translocons in a Ca2+ permeable state. Biochem. J. 478, 4005–4024 (2021).
Gronberg, A. et al. Antioxidants protect keratinocytes against M. ulcerans mycolactone cytotoxicity. PLoS ONE 5, e13839 (2010).
Kwaffo, Y. A. et al. Natural antioxidants attenuate mycolactone toxicity to RAW 264.7 macrophages. Exp. Biol. Med. 246, 1884–1894 (2021).
Guenin-Mace, L. et al. Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation. J. Clin. Invest. 123, 1501–1512 (2013).
George, K. M., Pascopella, L., Welty, D. M. & Small, P. L. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68, 877–883 (2000).
Simmonds, R. E., Lali, F. V., Smallie, T., Small, P. L. & Foxwell, B. M. Mycolactone inhibits monocyte cytokine production by a posttranscriptional mechanism. J. Immunol. 182, 2194–2202 (2009).
Coutanceau, E. et al. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell Microbiol. 7, 1187–1196 (2005).
Acknowledgements
The authors thank the individuals affected by Buruli ulcer for their contribution in Box 3.
Author information
Authors and Affiliations
Contributions
Introduction (R.R.Y., R.E.S. and R.O.P.); Epidemiology (R.R.Y., R.E.S., P.D.R.J., S.E., K.A. and G.P.); Mechanisms/pathophysiology (R.R.Y., R.E.S., G.P. and S.E.); Diagnosis, screening and prevention (R.R.Y., R.E.S., D.K.deS., P.D.R.J. and G.P.); Management (R.R.Y., R.E.S., D.K.deS., P.D.R.J., Y.A.A., R.O.P. and S.S.); Quality of life (R.R.Y., Y.A.A., R.O.P. and P.D.R.J.); Outlook (all authors).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks R. Gozlan, B. C. De Jong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Informed consent
The authors affirm that patient participants provided informed consent for publication of their experiences in Box 3. The authors affirm that human research participants provided informed consent for publication of the images in Fig. 5.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yotsu, R.R., Simmonds, R.E., de Souza, D.K. et al. Buruli ulcer. Nat Rev Dis Primers 11, 89 (2025). https://doi.org/10.1038/s41572-025-00672-9
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-025-00672-9