Abstract
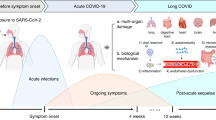
Long COVID is an infection-associated chronic condition that typically occurs within 3 months of acute COVID-19 infection in which symptoms are intermittently or continuously present for at least 3 months. Long COVID is estimated to affect between 80 and 400 million people globally, with an incidence of 5–20% in the community and up to 50% among hospitalized patients following acute SARS-CoV-2 infection. Common neuropsychiatric and mental health symptoms of long COVID include memory deficits, executive dysfunction, anxiety, depression, recurring headaches, sleep disturbances, neuropathies, problems with taste and smell, and dizziness that accompanies erratic heart rates and severe post-exertional malaise. Underlying pathophysiological mechanisms includes SARS-CoV-2 viral persistence, herpesvirus reactivation, microbiota dysbiosis, autoimmunity, clotting and endothelial abnormalities, and chronic immune activation. Owing to the variability in the clinical presentation, management must be tailored based on a patient’s presenting symptoms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yong, S. J. & Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 32, e2315 (2022).
Sivan, M. & Taylor, S. NICE guideline on long covid. BMJ 371, m4938 (2020).
Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22, e102–e107 (2022).
Ballering, A. V., van Zon, S. K. R., Olde Hartman, T. C., Rosmalen, J. G. M. & Lifelines Corona Research, I. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 400, 452–461 (2022).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Altmann, D. M., Whettlock, E. M., Liu, S., Arachchillage, D. J. & Boyton, R. J. The immunology of long COVID. Nat. Rev. Immunol. 23, 618–634 (2023). This study reviews the immunology of long COVID.
Al-Aly, Z., Bowe, B. & Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28, 1461–1467 (2022).
Whitaker, M. et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat. Commun. 13, 1957 (2022).
Office for National Statistics. Self-reported long COVID after two doses of a coronavirus (COVID-19) vaccine in the UK: 26 January 2022 (Office for National Statistics, 2022).
Fernandez-de-Las-Penas, C. et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses 14, 2629 (2022).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023). This study reviews mechanisms of long COVID.
Woldegiorgis, M. et al. Long COVID in a highly vaccinated but largely unexposed Australian population following the 2022 SARS-CoV-2 Omicron wave: a cross-sectional survey. Med. J. Aust. 220, 323–330 (2024).
Nguyen, K. H. et al. Prevalence and factors associated with long COVID symptoms among U.S. adults, 2022. Vaccines 12, 99 (2024).
Ayoubkhani, D. et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 377, e069676 (2022).
Rogers, J. P. et al. Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J. Neurol. Neurosurg. Psychiatry 92, 932–941 (2021).
Nepal, G. et al. Neurological manifestations of COVID-19: a systematic review. Crit. Care 24, 421 (2020).
Han, Y. et al. Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions. Transl. Psychiatry 11, 499 (2021).
Chou, S. H. et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw. Open. 4, e2112131 (2021).
Palaiodimou, L. et al. Prevalence, clinical characteristics and outcomes of Guillain–Barré syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur. J. Neurol. 28, 3517–3529 (2021).
Graham, E. L. et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 8, 1073–1085 (2021).
Ahmet’yanov, M. A., Reikhert, L. I., Kicherova, O. A., Veeva, D. M. & Makarova, D. V. Sleep disorders in patients after COVID-19. Neurosci. Behav. Physiol. 52, 645–648 (2022).
Tedjasukmana, R., Budikayanti, A., Islamiyah, W. R., Witjaksono, A. & Hakim, M. Sleep disturbance in post COVID-19 conditions: prevalence and quality of life. Front. Neurol. 13, 1095606 (2022).
Stefanou, M. I. et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther. Adv. Chronic Dis. 13, 20406223221076890 (2022).
Ceban, F. et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135 (2022).
Nasserie, T., Hittle, M. & Goodman, S. N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw. Open. 4, e2111417 (2021).
Hampshire, A. et al. Cognition and memory after covid-19 in a large community sample. N. Engl. J. Med. 390, 806–818 (2024).
Taquet, M. et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 9, 815–827 (2022).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29, 2347–2357 (2023).
Ziauddeen, N. et al. Characteristics and impact of long covid: findings from an online survey. PLoS ONE 17, e0264331 (2022).
Kromydas, T. et al. Occupational differences in the prevalence and severity of long-COVID: analysis of the coronavirus (COVID-19) infection survey. Occup. Env. Med. 80, 545–552 (2023).
Leone, M. A. et al. Outcome predictors of post-COVID conditions in the European academy of neurology COVID-19 registry. J. Neurol. https://doi.org/10.1007/s00415-024-12212-8 (2024).
Bosworth, M. L. et al. Risk of new-onset long COVID following reinfection with severe acute respiratory syndrome coronavirus 2: a community-based cohort study. Open. Forum Infect. Dis. 10, ofad493 (2023).
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019 (2021).
Ballouz, T. et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ 381, e074425 (2023).
Giussani, G. et al. Prevalence and trajectories of post-COVID-19 neurological manifestations: a systematic review and meta-analysis. Neuroepidemiology 58, 120–133 (2024).
Proal, A. D. et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. 24, 1616–1627 (2023).
Yin, K. et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 25, 218–225 (2024).
Klein, J. et al. Distinguishing features of long COVID identified through immune profiling. Nature 623, 139–148 (2023).
Rong, Z. et al. Persistence of spike protein at the skull-meninges-brain axis may contribute to the neurological sequelae of COVID-19. Cell Host Microbe 32, 2112–2130.e10 (2024).
Wong, A. C. et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 186, 4851–4867.e20 (2023).
Yonker, L. M. et al. Viral spike antigen clearance and augmented recovery in children with post-COVID multisystem inflammatory syndrome treated with larazotide. Sci. Transl. Med. 17, eadu4284 (2025).
White, D. W., Suzanne Beard, R. & Barton, E. S. Immune modulation during latent herpesvirus infection. Immunol. Rev. 245, 189–208 (2012).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895.e20 (2022).
Peluso, M. J. et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J. Clin. Invest. 133, e163669 (2023).
Cervia-Hasler, C. et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science 383, eadg7942 (2024).
Ruiz-Pablos, M., Paiva, B. & Zabaleta, A. Epstein–Barr virus-acquired immunodeficiency in myalgic encephalomyelitis — is it present in long COVID? J. Transl. Med. 21, 633 (2023).
Liu, Q. et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 71, 544–552 (2022).
Guo, C. et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe 31, 288–304.e8 (2023).
Duan, W. X., Wang, F., Liu, J. Y. & Liu, C. F. Relationship between short-chain fatty acids and parkinson’s disease: a review from pathology to clinic. Neurosci. Bull. 40, 500–516 (2024).
Mann, E. R., Lam, Y. K. & Uhlig, H. H. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595 (2024).
Lau, R. I. et al. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 24, 256–265 (2024).
Bodansky, A. et al. Autoantigen profiling reveals a shared post-COVID signature in fully recovered and long COVID patients. JCI Insight 8, e169515 (2023).
Etter, M. M. et al. Severe neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: a prospective cross-sectional study. Nat. Commun. 13, 6777 (2022).
Seibert, F. S. et al. Severity of neurological long-COVID symptoms correlates with increased level of autoantibodies targeting vasoregulatory and autonomic nervous system receptors. Autoimmun. Rev. 22, 103445 (2023).
Almulla, A. F., Maes, M., Zhou, B., Al-Hakeim, H. K. & Vojdani, A. brain-targeted autoimmunity is strongly associated with long COVID and its chronic fatigue syndrome as well as its affective symptoms. J. Adv. Res. 75, 621–633 (2025).
Chen, H.-J. et al. Transfer of IgG from long COVID patients induces symptomology in mice. Preprint at bioRxiv https://doi.org/10.1101/2024.05.30.596590 (2024).
Santos Guedes de Sa, K. et al. A causal link between autoantibodies and neurological symptoms in long COVID. Preprint at medRxiv https://doi.org/10.1101/2024.06.18.24309100 (2024).
Stein, E. et al. Efficacy of repeated immunoadsorption in patients with post-COVID myalgic encephalomyelitis/chronic fatigue syndrome and elevated β2-adrenergic receptor autoantibodies: a prospective cohort study. Lancet Reg. Health Eur. 49, 101161 (2025).
Lee, M. H. et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain 145, 2555–2568 (2022).
Turner, S. et al. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 34, 321–344 (2023).
Almulla, A. F., Thipakorn, Y., Zhou, B., Vojdani, A. & Maes, M. Immune activation and immune-associated neurotoxicity in long-COVID: a systematic review and meta-analysis of 103 studies comprising 58 cytokines/chemokines/growth factors. Brain Behav. Immun. 122, 75–94 (2024).
Peluso, M. J. & Deeks, S. G. Mechanisms of long COVID and the path toward therapeutics. Cell 187, 5500–5529 (2024). This study reviews how mechanisms of long COVID may help us to understand a path towards future treatments.
Fernandez-Castaneda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185, 2452–2468.e16 (2022).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Petersen, M. et al. Brain imaging and neuropsychological assessment of individuals recovered from a mild to moderate SARS-CoV-2 infection. Proc. Natl Acad. Sci. USA 120, e2217232120 (2023).
Rua, C. et al. Quantitative susceptibility mapping at 7T in COVID-19: brainstem effects and outcome associations. Brain https://doi.org/10.1093/brain/awae215 (2024).
Fontes-Dantas, F. L. et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 42, 112189 (2023).
Martinez-Marmol, R. et al. SARS-CoV-2 infection and viral fusogens cause neuronal and glial fusion that compromises neuronal activity. Sci. Adv. 9, eadg2248 (2023).
Crunfli, F. et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc. Natl Acad. Sci. USA 119, e2200960119 (2022).
Andrews, M. G. et al. Tropism of SARS-CoV-2 for human cortical astrocytes. Proc. Natl Acad. Sci. USA 119, e2122236119 (2022).
Wang, C. et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell 28, 331–342.e5 (2021).
Savelieff, M. G., Feldman, E. L. & Stino, A. M. Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 infection. Neurobiol. Dis. 168, 105715 (2022).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Katsoularis, I., Fonseca-Rodriguez, O., Farrington, P., Lindmark, K. & Fors Connolly, A. M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 398, 599–607 (2021).
Knight, R. et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation 146, 892–906 (2022).
Kell, D. B., Laubscher, G. J. & Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem. J. 479, 537–559 (2022).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 28, 2406–2415 (2022).
Monje, M. & Iwasaki, A. The neurobiology of long COVID. Neuron 110, 3484–3496 (2022). This paper discusses the neurobiology of long COVID.
Wu, X. et al. Damage to endothelial barriers and its contribution to long COVID. Angiogenesis 27, 5–22 (2024).
Pretorius, E. et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/post-acute sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 21, 148 (2022).
Bulfamante, G. et al. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J. Neurol. 268, 4486–4491 (2021).
Matschke, J. et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 19, 919–929 (2020).
Zingaropoli, M. A. et al. Neuro-axonal damage and alteration of blood-brain barrier integrity in COVID-19 patients. Cells 11, 2480 (2022).
Kong, W. et al. Neuropilin-1 mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio 13, e0230822 (2022).
Rutkai, I. et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 13, 1745 (2022).
Bocci, T., Campiglio, L. & Priori, A. in NEUROLOGY OF COVID–19 [Internet] Ch. 13, 195–201 (Milano University Press, 2021).
Todisco, M. et al. Isolated bulbar palsy after SARS-CoV-2 infection. Lancet Neurol. 20, 169–170 (2021).
Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707 (2022).
Teller, N. et al. Feasibility of diffusion-tensor and correlated diffusion imaging for studying white-matter microstructural abnormalities: application in COVID-19. Hum. Brain Mapp. 44, 3998–4010 (2023).
Bispo, D. D. C. et al. Altered structural connectivity in olfactory disfunction after mild COVID-19 using probabilistic tractography. Sci. Rep. 13, 12886 (2023).
Esposito, F. et al. Olfactory loss and brain connectivity after COVID-19. Hum. Brain Mapp. 43, 1548–1560 (2022).
Bocci, T. et al. Brainstem clinical and neurophysiological involvement in COVID-19. J. Neurol. 268, 3598–3600 (2021). This study investigated other mechanisms in the neurophysiology of long COVID.
Manganelli, F. et al. Brainstem involvement and respiratory failure in COVID-19. Neurol. Sci. 41, 1663–1665 (2020).
Manganotti, P., Michelutti, M., Furlanis, G., Deodato, M. & Buoite Stella, A. Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clin. Neurophysiol. 151, 83–91 (2023).
Versace, V. et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. 145, 81–88 (2023).
Ortelli, P. et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19. Eur. J. Neurol. 29, 1652–1662 (2022).
Ferrucci, R. et al. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur. J. Neurol. 29, 2006–2014 (2022).
Cecchetti, G. et al. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J. Neurol. 269, 3400–3412 (2022).
York, E. M., Zhang, J., Choi, H. B. & MacVicar, B. A. Neuroinflammatory inhibition of synaptic long-term potentiation requires immunometabolic reprogramming of micro. Glia. Glia 69, 567–578 (2021).
Stratoulias, V. et al. ARG1-expressing microglia show a distinct molecular signature and modulate postnatal development and function of the mouse brain. Nat. Neurosci. 26, 1008–1020 (2023).
Bulfamante, G. et al. First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 86, 678–679 (2020).
Horsager, J. et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143, 3077–3088 (2020).
Emmi, A. et al. Detection of SARS-CoV-2 viral proteins and genomic sequences in human brainstem nuclei. NPJ Parkinsons Dis. 9, 25 (2023).
Dos Reis, R. S., Selvam, S. & Ayyavoo, V. Neuroinflammation in post COVID-19 sequelae: neuroinvasion and neuroimmune crosstalk. Rev. Med. Virol. 34, e70009 (2024).
Beardmore, R., Hou, R., Darekar, A., Holmes, C. & Boche, D. The locus coeruleus in aging and Alzheimer’s disease: a postmortem and brain imaging review. J. Alzheimers Dis. 83, 5–22 (2021).
Yang, A. C. et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595, 565–571 (2021).
Brundin, P., Nath, A. & Beckham, J. D. Is COVID-19 a perfect storm for Parkinson’s disease? Trends Neurosci. 43, 931–933 (2020).
Beauchamp, L. C., Finkelstein, D. I., Bush, A. I., Evans, A. H. & Barnham, K. J. Parkinsonism as a third wave of the COVID-19 pandemic? J. Parkinsons Dis. 10, 1343–1353 (2020).
Calculli, A. et al. Parkinson disease following COVID-19: report of six cases. Eur. J. Neurol. 30, 1272–1280 (2023).
Ferrucci, R. et al. Brain positron emission tomography (PET) and cognitive abnormalities one year after COVID-19. J. Neurol. 270, 1823–1834 (2023).
Zilio, G. et al. SARS-CoV-2-mimicking pseudoviral particles accelerate α-synuclein aggregation in vitro. ACS Chem. Neurosci. 15, 215–221 (2024).
Centers for Disease Control and Prevention. Clinical overview of long COVID. CDC https://www.cdc.gov/long-covid/hcp/clinical-overview/index.html (2025).
World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus: 6 October 2021 https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (WHO, 2021).
National Academies of Sciences, Engineering, and Medicine. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences (National Academies Press, 2024).
Ely, E. W., Brown, L. M. & Fineberg, H. V. Long Covid defined. N. Engl. J. Med. 391, 1746–1753 (2024). Both this article and the National Academies of Sciences, Engineering, and Medicine article provide working definitions of long COVID.
Greenhalgh, T., Sivan, M., Delaney, B., Evans, R. & Milne, R. Long covid — an update for primary care. BMJ 378, e072117 (2022).
Clutterbuck, D. et al. Barriers to healthcare access and experiences of stigma: findings from a coproduced long covid case-finding study. Health Expect. 27, e14037 (2024).
Baz, S. A. et al. Long COVID and health inequalities: what’s next for research and policy advocacy? Health Expect. 27, e70047 (2024).
Kenny, G. et al. Identification of distinct long COVID clinical phenotypes through cluster analysis of self-reported symptoms. Open. Forum Infect. Dis. 9, ofac060 (2022).
Gentilotti, E. et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine 62, 102107 (2023).
Dagliati, A. et al. Characterization of long COVID temporal sub-phenotypes by distributed representation learning from electronic health record data: a cohort study. EClinicalMedicine 64, 102210 (2023).
Gottlieb, M. et al. Long COVID clinical phenotypes up to 6 months after infection identified by latent class analysis of self-reported symptoms. Open. Forum Infect. Dis. 10, ofad277 (2023).
Kisiel, M. A. et al. Clustering analysis identified three long COVID phenotypes and their association with general health status and working ability. J. Clin. Med. 12, 3617 (2023).
Kitsios, G. D. et al. Subphenotypes of self-reported symptoms and outcomes in long COVID: a prospective cohort study with latent class analysis. BMJ Open. 14, e077869 (2024).
Liew, F. et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat. Immunol. 25, 607–621 (2024).
Espin, E. et al. Cellular and molecular biomarkers of long COVID: a scoping review. EBioMedicine 91, 104552 (2023).
Lai, Y. J. et al. Biomarkers in long COVID-19: a systematic review. Front. Med. 10, 1085988 (2023).
Patel, M. A. et al. Organ and cell-specific biomarkers of long-COVID identified with targeted proteomics and machine learning. Mol. Med. 29, 26 (2023).
Patel, M. A. et al. Elevated vascular transformation blood biomarkers in long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol. Med. 28, 122 (2022).
Chioh, F. W. et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife 10, e64909 (2021).
Iosef, C. et al. Plasma proteome of long-COVID patients indicates HIF-mediated vasculo-proliferative disease with impact on brain and heart function. J. Transl. Med. 21, 377 (2023).
Zanoli, L. et al. Vascular dysfunction of COVID-19 is partially reverted in the long-term. Circ. Res. 130, 1276–1285 (2022).
Szeghy, R. E. et al. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J. Appl. Physiol. 132, 1297–1309 (2022).
Santoro, L. et al. Impaired endothelial function in convalescent phase of COVID-19: A 3 month follow up observational prospective study. J. Clin. Med. 11, 1774 (2022).
Alhuthail, E., Stockley, J., Coney, A. & Cooper, B. Measurement of breathing in patients with post-COVID-19 using structured light plethysmography (SLP). BMJ Open. Respir. Res. 8, e001070 (2021).
Bazdar, S. et al. A systematic review of chest imaging findings in long COVID patients. J. Pers. Med. 13, 282 (2023).
Matheson, A. M. et al. Persistent 129Xe MRI pulmonary and CT vascular abnormalities in symptomatic individuals with post-acute COVID-19 syndrome. Radiology 305, 466–476 (2022).
Hugon, J. et al. Cognitive decline and brainstem hypometabolism in long COVID: a case series. Brain Behav. 12, e2513 (2022).
Martini, A. L. et al. Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur. J. Nucl. Med. Mol. Imaging 50, 90–102 (2022).
Heine, J. et al. Structural brain changes in patients with post-COVID fatigue: a prospective observational study. EClinicalMedicine 58, 101874 (2023).
Hellgren, L. et al. Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open. 11, e055164 (2021).
Golding, L. et al. A novel anti-nucleocapsid antibody avidity method for identifying SARS-CoV-2 reinfections. J. Infect. Dis. https://doi.org/10.1093/infdis/jiae072 (2024).
Peluso, M. J. et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci. Adv. 7, eabh3409 (2021).
Peluso, M. J. et al. Plasma-based antigen persistence in the post-acute phase of COVID-19. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(24)00211-1 (2024).
Siso-Almirall, A. et al. Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int. J. Env. Res. Public. Health 18, 4350 (2021).
Yelin, D. et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin. Microbiol. Infect. 28, 955–972 (2022).
Thornton, G. M. et al. The impact of heating, ventilation, and air conditioning design features on the transmission of viruses, including the 2019 novel coronavirus: a systematic review of ventilation and coronavirus. PLoS Glob. Public. Health 2, e0000552 (2022).
Nejatian, A. et al. How much natural ventilation rate can suppress COVID-19 transmission in occupancy zones? J. Res. Med. Sci. 28, 84 (2023).
Floriano, I. et al. Effectiveness of wearing masks during the COVID-19 outbreak in cohort and case-control studies: a systematic review and meta-analysis. J. Bras. Pneumol. 49, e20230003 (2024).
Bowe, B., Xie, Y. & Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 28, 2398–2405 (2022).
Azzolini, E. et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA https://doi.org/10.1001/jama.2022.11691 (2022).
Xie, Y., Choi, T. & Al-Aly, Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of covid-19: cohort study. BMJ 381, e074572 (2023).
Durstenfeld, M. S. et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent long COVID symptoms in an observational cohort study. J. Med. Virol. 96, e29333 (2024).
Ioannou, G. N. et al. Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. veterans : a target trial emulation. Ann. Intern. Med. 176, 1486–1497 (2023).
Brannock, M. D. et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat. Commun. 14, 2914 (2023).
Ceban, F. et al. COVID-19 vaccination for the prevention and treatment of long COVID: a systematic review and meta-analysis. Brain Behav. Immun. 111, 211–229 (2023).
Watanabe, A., Iwagami, M., Yasuhara, J., Takagi, H. & Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis. Vaccine 41, 1783–1790 (2023).
The Lancet Infectious Diseases Where are the long COVID trials? Lancet Infect. Dis. 23, 879 (2023).
Chee, Y. J., Fan, B. E., Young, B. E., Dalan, R. & Lye, D. C. Clinical trials on the pharmacological treatment of long COVID: a systematic review. J. Med. Virol. 95, e28289 (2023).
Shah, W., Hillman, T., Playford, E. D. & Hishmeh, L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372, n136 (2021).
Seo, J. W. et al. Updated clinical practice guidelines for the diagnosis and management of long COVID. Infect. Chemother. 56, 122–157 (2024).
Jacobs, M. M., Evans, E. & Ellis, C. Racial, ethnic, and sex disparities in the incidence and cognitive symptomology of long COVID-19. J. Natl Med. Assoc. 115, 233–243 (2023).
Perlis, R. H. et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw. Open. 5, e2238804 (2022).
Stussman, B. et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front. Neurol. 11, 1025 (2020).
Haunhorst, S. et al. Towards an understanding of physical activity-induced post-exertional malaise: insights into microvascular alterations and immunometabolic interactions in post-COVID condition and myalgic encephalomyelitis/chronic fatigue syndrome. Infection https://doi.org/10.1007/s15010-024-02386-8 (2024).
Kos, D. et al. Activity pacing self-management in chronic fatigue syndrome: a randomized controlled trial. Am. J. Occup. Ther. 69, 6905290020 (2015).
Tyson, S. F. Appraisal of Clinical Practice Guideline: National Institute for Health and Care Excellence (NICE) clinical practice guideline for myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management. J. Physiother. 70, 155 (2024).
Kuut, T. A. et al. Efficacy of cognitive-behavioral therapy targeting severe fatigue following coronavirus disease 2019: results of a randomized controlled trial. Clin. Infect. Dis. 77, 687–695 (2023).
Oliver-Mas, S. et al. Transcranial direct current stimulation for post-COVID fatigue: a randomized, double-blind, controlled pilot study. Brain Commun. 5, fcad117 (2023).
Santana, K. et al. Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. 16, 100–107 (2023).
Robbins, T. et al. Hyperbaric oxygen therapy for the treatment of long COVID: early evaluation of a highly promising intervention. Clin. Med. 21, e629–e632 (2021).
Hawkins, J., Hires, C., Keenan, L. & Dunne, E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: a randomized, double-blinded, placebo controlled clinical trial. Complement. Ther. Med. 67, 102823 (2022).
Reitsma, L., Boelen, P. A., de Keijser, J. & Lenferink, L. I. M. Self-guided online treatment of disturbed grief, posttraumatic stress, and depression in adults bereaved during the COVID-19 pandemic: a randomized controlled trial. Behav. Res. Ther. 163, 104286 (2023).
Hausswirth, C., Schmit, C., Rougier, Y. & Coste, A. Positive impacts of a four-week neuro-meditation program on cognitive function in post-acute sequelae of COVID-19 patients: a randomized controlled trial. Int. J. Env. Res. Public. Health 20, 1361 (2023).
World Health Organization. Clinical management of COVID-19: living guideline (WHO, 2025).
Bahar-Fuchs, A., Martyr, A., Goh, A. M., Sabates, J. & Clare, L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst. Rev. 3, CD013069 (2019).
Simon, S. S., Yokomizo, J. E. & Bottino, C. M. Cognitive intervention in amnestic mild cognitive impairment: a systematic review. Neurosci. Biobehav. Rev. 36, 1163–1178 (2012).
Kim, E. J. et al. Current status of cognitive remediation for psychiatric disorders: a review. Front. Psychiatry 9, 461 (2018).
Palladini, M. et al. Cognitive remediation therapy for post-acute persistent cognitive deficits in COVID-19 survivors: a proof-of-concept study. Neuropsychol. Rehabil. 33, 1207–1224 (2023).
Zilberman-Itskovich, S. et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci. Rep. 12, 11252 (2022).
Reijnders, J., van Heugten, C. & van Boxtel, M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res. Rev. 12, 263–275 (2013).
Sanjuan, M., Navarro, E. & Calero, M. D. Effectiveness of cognitive interventions in older adults: a review. Eur. J. Investig. Health Psychol. Educ. 10, 876–898 (2020).
Kaufmann, J., Gould, O. & Lloyd, V. Seeking care for long COVID: a narrative analysis of Canadian experiences. J. Patient Exp. 10, 23743735231151770 (2023).
Ziauddeen NA, P. M., O’hara, M. E., Hastie, C. & Alwan, N. A. Symptom patterns and triggers of long covid: findings from a longitudinal online survey. Eur. J. Public. Health https://doi.org/10.1093/eurpub/ckad160.091 (2023).
Mastrorosa, I. et al. What is the impact of post-COVID-19 syndrome on health-related quality of life and associated factors: a cross-sectional analysis. Health Qual. Life Outcomes 21, 28 (2023).
Al-Jabr, H., Thompson, D. R., Castle, D. J. & Ski, C. F. Experiences of people with long COVID: symptoms, support strategies and the long COVID optimal health programme (LC-OHP). Health Expect. 27, 13879 (2023).
Perlis, R. H. et al. Association of post-COVID-19 condition symptoms and employment status. JAMA Netw. Open. 6, e2256152 (2023).
Office for National Statistics. Self-Reported Coronavirus (COVID-19) Infections and Associated Symptoms, England and Scotland https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/selfreportedcoronaviruscovid19infectionsandassociatedsymptomsenglandandscotland/november2023tomarch2024 (Office for National Statistics, 2024).
Ramos, S. C., Maldonado, J. E., Vandeplas, A., Vanyolos, I. Long COVID: A Tentative Assessment of its Impact on Labour Market Participation and Potential Economic Effects in the EU https://economy-finance.ec.europa.eu/publications/long-covid-tentative-assessment-its-impact-labour-market-participation-and-potential-economic_en, (European Commission, 2024).
Ayoubkhani, D. et al. Employment outcomes of people with Long Covid symptoms: community-based cohort study. Eur. J. Public. Health 34, 489–496 (2024).
Ziauddeen, N., Pantelic, M., O’Hara, M. E., Hastie, C. & Alwan, N. A. Impact of long COVID-19 on work: a co-produced survey. Lancet 402, S98 (2023). This paper discusses the impact of long COVID on patients.
O’Brien, K. K. et al. Conceptualising the episodic nature of disability among adults living with Long COVID: a qualitative study. BMJ Glob. Health 8, e011276 (2023).
Shabnam, S. et al. Socioeconomic inequalities of long COVID: a retrospective population-based cohort study in the United Kingdom. J. R. Soc. Med. 116, 263–273 (2023).
Cheetham, N. J. et al. Social determinants of recovery from ongoing symptoms following COVID-19 in two UK longitudinal studies: a prospective cohort study. BMJ Public. Health 3, e001166 (2025).
Chilunga, F. P. et al. Differences in incidence, nature of symptoms, and duration of long COVID among hospitalised migrant and non-migrant patients in the Netherlands: a retrospective cohort study. Lancet Reg. Health Eur. 29, 100630 (2023).
Tanne, J. H. Covid-19: US studies show racial and ethnic disparities in long covid. BMJ 380, 535 (2023).
McGreevy, A. et al. Ethnic inequalities in the impact of COVID-19 on primary care consultations: a time series analysis of 460,084 individuals with multimorbidity in South London. BMC Med. 21, 26 (2023).
Woodrow, M., Ziauddeen, N., Smith, D. & Alwan, N. A. Exploring long covid prevalence and patient uncertainty by sociodemographic characteristics using GP patient survey data. Health Expect. 28, e70202 (2025).
Carlile, O. et al. Impact of long COVID on health-related quality-of-life: an OpenSAFELY population cohort study using patient-reported outcome measures (OpenPROMPT). Lancet Reg. Health Eur. 40, 100908 (2024).
Poudel, A. N. et al. Impact of Covid-19 on health-related quality of life of patients: a structured review. PLoS ONE 16, e0259164 (2021).
Baz, S. A., Fang, C., Carpentieri, J. D. & Sheard, L. ‘I don’t know what to do or where to go’. Experiences of accessing healthcare support from the perspectives of people living with long covid and healthcare professionals: a qualitative study in Bradford, UK. Health Expect. 26, 542–554 (2023).
Pantelic, M. et al. Long covid stigma: estimating burden and validating scale in a UK-based sample. PLoS ONE 17, e0277317 (2022).
Buonsenso, D. et al. Social stigma in children with long COVID. Children 10, 1518 (2023).
Scholz, U., Bierbauer, W. & Luscher, J. Social stigma, mental health, stress, and health-related quality of life in people with long COVID. Int. J. Env. Res. Public. Health 20, 3927 (2023).
Nyaaba, G. N. et al. Experiences of stigma and access to care among long COVID patients: a qualitative study in a multi-ethnic population in the Netherlands. BMJ Open. 15, e094487 (2025).
Smyth, N. et al. People from ethnic minorities seeking help for long COVID: a qualitative study. Br. J. Gen. Pract. 74, e814–e822 (2024).
Arienti, C. et al. Rehabilitation and COVID-19: systematic review by Cochrane Rehabilitation. Eur. J. Phys. Rehabil. Med. 59, 800–818 (2023).
Frontera, J. A. et al. Evaluation and treatment approaches for neurological post-acute sequelae of COVID-19: a consensus statement and scoping review from the Global COVID-19 Neuro Research Coalition. J. Neurol. Sci. 454, 120827 (2023).
Fine, J. S. et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cognitive symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R 14, 96–111 (2022).
Guttuso, T. Jr., Zhu, J. & Wilding, G. E. Lithium aspartate for long COVID fatigue and cognitive dysfunction: a randomized clinical trial. JAMA Netw. Open. 7, e2436874 (2024).
Kwan, A. T. H. et al. Impacts of metabolic disruption, body mass index and inflammation on cognitive function in post-COVID-19 condition: a randomized controlled trial on vortioxetine. Ann. Gen. Psychiatry 23, 10 (2024).
Lupfer, C. R., Nadler, R., Amen, R. & Martin, A. Inhalation of sodium pyruvate to reduce the symptoms and severity of respiratory diseases including COVID-19, long COVID, and pulmonary fibrosis. Eur. J. Respiratory Med. 3, 229–237 (2021).
Thurgur, H. et al. Feasibility of a cannabidiol-dominant cannabis-based medicinal product for the treatment of long COVID symptoms: a single-arm open-label feasibility trial. Br. J. Clin. Pharmacol. 90, 1081–1093 (2024).
Finnigan, L. E. M. et al. Efficacy and tolerability of an endogenous metabolic modulator (AXA1125) in fatigue-predominant long COVID: a single-centre, double-blind, randomised controlled phase 2a pilot study. EClinicalMedicine 59, 101946 (2023).
Isman, A. et al. Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19. Brain Behav. Immun. Health 36, 100733 (2024).
Geng, L. N. et al. Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial. JAMA Intern. Med. 184, 1024–1034 (2024).
Viner, R. M. et al. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch. Dis. Child. 106, 802–807 (2021).
Consiglio, C. R. et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 183, 968–981.e7 (2020).
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Fraser, D. D., Patterson, E. K., Daley, M. & Cepinskas, G. Inflammation and endothelial injury profiling of COVID-19 pediatric multisystem inflammatory syndrome (MIS-C). Front. Pediatr. 9, 597926 (2021).
Patel, M. A. et al. The plasma proteome differentiates the multisystem inflammatory syndrome in children (MIS-C) from children with SARS-CoV-2 negative sepsis. Mol. Med. 30, 51 (2024).
Castagnoli, R. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatrics 174, 882–889 (2020).
Ha, E. K., Kim, J. H. & Han, M. Y. Long COVID in children and adolescents: prevalence, clinical manifestations, and management strategies. Clin. Exp. Pediatr. 66, 465–474 (2023).
Borch, L. et al. Long COVID symptoms and duration in SARS-CoV-2 positive children — a nationwide cohort study. Eur. J. Pediatrics https://doi.org/10.1007/s00431-021-04345-z (2022).
Fainardi, V. et al. Long COVID in children and adolescents. Life 12, 285–285 (2022).
Izquierdo-Pujol, J. et al. Post COVID-19 condition in children and adolescents: an emerging problem. Front. Pediatrics 10, 894204 (2022).
Kostev, K. et al. Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19. Pediatr. Res. https://doi.org/10.1038/s41390-022-02111-x (2022).
Heiss, R. et al. Pulmonary dysfunction after pediatric COVID-19. Radiology 306, e221250 (2023).
Cocciolillo, F. et al. Orbito-frontal cortex hypometabolism in children with post-COVID condition (Long COVID): a preliminary experience. Pediatr. Infect. Dis. J. 41, 663–665 (2022).
Morand, A. et al. Similar patterns of [18F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: a paediatric case series. Eur. J. Nucl. Med. Mol. Imaging 49, 913–920 (2022).
Lopez-Leon, S. et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci. Rep. 12, 9950 (2022).
Camporesi, A. et al. Characteristics and predictors of Long Covid in children: a 3-year prospective cohort study. EClinicalMedicine 76, 102815 (2024).
Acknowledgements
J.E.W. and E.W.E. are supported by NIA 1R01AG085873 and by the Department of Veterans Affairs Geriatric Research, Education and Clinical Center. M.J.P. is supported on K23AI157875 and 1R01NS136197. E.W.E. is supported by the National Institute on Aging (NIA) R01AG058639, NIA 1R01AG085873 and VA Merit 1RX002992. A.I. is supported by the Else Kröner Fresenius Prize for Medical Research 2023, grants from National Institute of Allergy and Infectious Disease (NIAID) R01AI157488, the Howard Hughes Medical Institute Collaborative COVID-19 Initiative, the Howard Hughes Medical Institute Emerging Pathogens Initiative, and the Howard Hughes Medical Institute. C.L.Y. is supported by The Brazilian National Council for Scientific and Technological Development (CNPQ 403307/2021-0, 445340/2024-0, 315953/2021-7). D.D.F. is supported by the Canadian Institutes for Health Research (grant no. 185352, Long COVID Web) and the Schmidt Initiative for Long Covid (SILC-2023-006, LC-Optimize and SILC-2024-004, LC-Revitalize, NCT06928272).
Author information
Authors and Affiliations
Contributions
All authors contributed to all sections of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.E.W. received research support from the Department of Veterans Affairs Office of Rural Health, Ac-Immune, IONIS therapeutics, Bristol Meyers Squibb and Ono therapeutics outside the submitted work. M.J.P. received consulting fees from Gilead Sciences, AstraZeneca, BioVie, Apellis Pharmaceuticals and BioNTech, and research support from Aerium Therapeutics and Shionogi, outside the submitted work. R.H. received grant funding from Fresenius Kabi Germany and Austrian Science Fund; and has received payments or honoraria from BD, Integra, Neuroptics and Zoll, not related to the submitted work. N.A.A. is a Long Covid Kids Charity Champion, is a scientific adviser to the Long Covid Support Charity, and has contributed in an advisory capacity to WHO and the EU Commission’s Expert Panel on effective ways of investing in health meetings in relation to post-COVID-19 conditions. E.W.E. has NIH funding for an ongoing clinical trial of immunomodulation in long COVID for which the JAK–STAT medical intervention is donated by Eli Lilly; E.W.E. has no financial relationship with Eli Lilly and has no stocks or paid consultancies with Eli Lilly. C.L.Y. is supported by CNPQ. D.G., S.O., D.D.F., S.R.F., A.I., T.B., A.P. and D.A. declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks N. Babel; D. Marazziti; and B. Michael, who co-reviewed with R. Matthews, for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wilson, J.E., Gurdasani, D., Helbok, R. et al. COVID-19-associated neurological and psychological manifestations. Nat Rev Dis Primers 11, 91 (2025). https://doi.org/10.1038/s41572-025-00674-7
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-025-00674-7