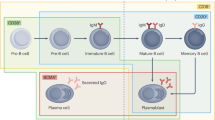
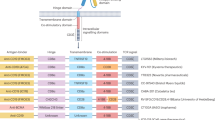
Abstract
Chimeric antigen receptor (CAR) T cell therapy has been highly effective in eradicating malignant B cells in cancer, and this success has prompted an extension of the approach to areas beyond oncology. In pioneering studies, CAR T cells targeting the B cell marker CD19 demonstrated robust efficacy as treatment for the autoimmune disease systemic lupus erythematosus. Patients who received anti-CD19 CAR T cells experienced remission of most or all clinical manifestations and discontinued prior medications. These results have spurred intense interest in extending these observations to larger patient cohorts and other autoimmune conditions. More nuanced strategies for use of CARs in autoimmunity have also been developed. Here, we offer insight into the role of B cells in the pathophysiology of autoimmunity and present an overview of preclinical studies and clinical trials that use engineered cell therapy for autoimmune disorders. In discussing the prospects and challenges of this emerging field, a view emerges in which the promise of clinical efficacy invites careful consideration of potential pitfalls.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Riddell, S. R., Jensen, M. C. & June, C. H. Chimeric antigen receptor-modified T cells: clinical translation in stem cell transplantation and beyond. Biol. Blood Marrow Transplant. 19, S2–S5 (2013).
Sadelain, M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 125, 3392–3400 (2015).
Scheuermann, R. H. & Racila, E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk. Lymphoma 18, 385–397 (1995).
Shah, N., Chari, A., Scott, E., Mezzi, K. & Usmani, S. Z. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 34, 985–1005 (2020).
Goyco Vera, D., Waghela, H., Nuh, M., Pan, J. & Lulla, P. Approved CAR-T therapies have reproducible efficacy and safety in clinical practice. Hum. Vaccin. Immunother. 20, 2378543 (2024).
Bhaskar, S. T., Dholaria, B., Savani, B. N., Sengsayadeth, S. & Oluwole, O. Overview of approved CAR-T products and utility in clinical practice. Clin. Hematol. Int. 6, 93–99 (2024).
Shah, N. N. & Sokol, L. Targeting CD22 for the treatment of B-cell malignancies. Immunotargets Ther. 10, 225–236 (2021).
Abramson, J. S. et al. Two-year follow-up of lisocabtagene maraleucel in relapsed or refractory large B-cell lymphoma in TRANSCEND NHL 001. Blood 143, 404–416 (2024).
Curran, K. J. et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134, 2361–2368 (2019).
Fowler, N. H. et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat. Med. 28, 325–332 (2022).
Gardner, R. A. et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129, 3322–3331 (2017).
Laetsch, T. W. et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA Trial. J. Clin. Oncol. 41, 1664–1669 (2023).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Neelapu, S. S. et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 141, 2307–2315 (2023).
Park, J. H., Geyer, M. B. & Brentjens, R. J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 127, 3312–3320 (2016).
Schultz, L. M. et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J. Clin. Oncol. 40, 945–955 (2022).
Schuster, S. J. et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 22, 1403–1415 (2021).
Shah, N. N. et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J. Clin. Oncol. 39, 1650–1659 (2021).
Talleur, A. et al. Preferential expansion of CD8+ CD19-CAR T cells postinfusion and the role of disease burden on outcome in pediatric B-ALL. Blood Adv. https://doi.org/10.1182/bloodadvances.2021006293 (2022).
Wang, M. et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J. Clin. Oncol. 41, 555–567 (2023).
Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62, 222–233 (2010).
Radic, M., Neeli, I. & Marion, T. Prospects for CAR T cell immunotherapy in autoimmune diseases: clues from lupus. Expert. Opin. Biol. Ther. 22, 499–507 (2022).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016). This work presents a notable approach to deplete antigen-specific B cells in a mouse model of pemphigus.
Kansal, R. et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 11, eaav1648 (2019). This work is the first report of symptom improvement and anti-CD19 CAR T cell persistence in two mouse models of lupus.
Ahuja, A. et al. An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in lupus. J. Immunol. 187, 3888–3894 (2011).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021). This study presents the first application of CD19 CAR T cell therapy in a patient with AID.
Muller, F. et al. Comparison of the safety profiles of CD19-targeting CAR T-cell therapy in patients with SLE and B-cell lymphoma. Blood https://doi.org/10.1182/blood.2025028375 (2025).
Cancro, M. P. & Tomayko, M. M. Memory B cells and plasma cells: the differentiative continuum of humoral immunity. Immunol. Rev. 303, 72–82 (2021).
Cyster, J. G. & Allen, C. D. C. B cell responses: cell interaction dynamics and decisions. Cell 177, 524–540 (2019).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 40, 413–442 (2022).
William, J., Euler, C., Christensen, S. & Shlomchik, M. J. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297, 2066–2070 (2002).
Di Niro, R. et al. Salmonella infection drives promiscuous B cell activation followed by extrafollicular affinity maturation. Immunity 43, 120–131 (2015).
Jenks, S. A. et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49, 725–739.e6 (2018). This work shows that extrafollicular B cell responses in SLE are associated with overactivity of TLR7 signalling in activated naive B cells.
Woodruff, M. C. et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 611, 139–147 (2022).
Mouat, I. C., Goldberg, E. & Horwitz, M. S. Age-associated B cells in autoimmune diseases. Cell Mol. Life Sci. 79, 402 (2022).
Sanz, I. et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol. 10, 2458 (2019).
Johnson, J. L., Scholz, J. L., Marshak-Rothstein, A. & Cancro, M. P. Molecular pattern recognition in peripheral B cell tolerance: lessons from age-associated B cells. Curr. Opin. Immunol. 61, 33–38 (2019).
Deguine, J. & Xavier, R. J. B cell tolerance and autoimmunity: lessons from repertoires. J. Exp. Med. 221, e20231314 (2024).
Dorner, T., Jacobi, A. M. & Lipsky, P. E. B cells in autoimmunity. Arthritis Res. Ther. 11, 247 (2009).
Meng, W. et al. An atlas of B-cell clonal distribution in the human body. Nat. Biotechnol. 35, 879–884 (2017).
Tipton, C. M. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Ziegler, A. G. et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309, 2473–2479 (2013).
Amagai, M., Koch, P. J., Nishikawa, T. & Stanley, J. R. Pemphigus vulgaris antigen (desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J. Invest. Dermatol. 106, 351–355 (1996).
Harrington, W. J., Minnich, V., Hollingsworth, J. W. & Moore, C. V. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J. Lab. Clin. Med. 38, 1–10 (1951).
Woods, V. L. Jr., Oh, E. H., Mason, D. & McMillan, R. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood 63, 368–375 (1984).
Agrawal, S., Misra, R. & Aggarwal, A. Autoantibodies in rheumatoid arthritis: association with severity of disease in established RA. Clin. Rheumatol. 26, 201–204 (2007).
Lerner, R. A., Glassock, R. J. & Dixon, F. J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J. Exp. Med. 126, 989–1004 (1967).
Clynes, R., Dumitru, C. & Ravetch, J. V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052–1054 (1998).
Patrick, J. & Lindstrom, J. Autoimmune response to acetylcholine receptor. Science 180, 871–872 (1973).
Chan, O. T., Hannum, L. G., Haberman, A. M., Madaio, M. P. & Shlomchik, M. J. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 189, 1639–1648 (1999).
Noorchashm, H. et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet β-cells of nonobese diabetic mice. J. Immunol. 163, 743–750 (1999).
Guerrier, T. et al. Proinflammatory B-cell profile in the early phases of MS predicts an active disease. Neurol. Neuroimmunol. Neuroinflamm 5, e431 (2018).
Li, R. et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci. Transl. Med. 7, 310ra166 (2015).
Muller, F. et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Siegel, C. H. & Sammaritano, L. R. Systemic lupus erythematosus: a review. JAMA 331, 1480–1491 (2024).
Jenks, S. A. et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 52, 203 (2020).
Jego, G. et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 (2003).
Hu, Z. et al. BCMA-targeted CAR T cell therapy can effectively induce disease remission in refractory lupus nephritis. Ann. Rheum. Dis. https://doi.org/10.1016/j.ard.2025.06.2128 (2025).
Sokolova, M. V., Schett, G. & Steffen, U. Autoantibodies in rheumatoid arthritis: historical background and novel findings. Clin. Rev. Allergy Immunol. 63, 138–151 (2022).
Meednu, N. et al. Dynamic spectrum of ectopic lymphoid B cell activation and hypermutation in the RA synovium characterized by NR4A nuclear receptor expression. Cell Rep. 39, 110766 (2022).
Bucci, L. et al. Bispecific T cell engager therapy for refractory rheumatoid arthritis. Nat. Med. 30, 1593–1601 (2024). This study reports the use of recombinant bivalent antibodies for effective RA treatment.
Rech, J. et al. Abatacept inhibits inflammation and onset of rheumatoid arthritis in individuals at high risk (ARIAA): a randomised, international, multicentre, double-blind, placebo-controlled trial. Lancet 403, 850–859 (2024).
Thoreau, B., Chaigne, B. & Mouthon, L. Role of B-cell in the pathogenesis of systemic sclerosis. Front. Immunol. 13, 933468 (2022).
Bosello, S. et al. B cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trial. Arthritis Res. Ther. 12, R54 (2010).
Distler, J. H. W. et al. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 15, 705–730 (2019).
Gunther, J. et al. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res. Ther. 16, R65 (2014).
Ebata, S. et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 3, e489–e497 (2021).
Bergmann, C. et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann. Rheum. Dis. 82, 1117–1120 (2023).
Wang, X. et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell 187, 4890–4904.e9 (2024).
Lee, J. et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Invest. 130, 6317–6324 (2020).
Payne, A. S. et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J. Clin. Invest. 115, 888–899 (2005).
Mao, X., Sano, Y., Park, J. M. & Payne, A. S. p38 MAPK activation is downstream of the loss of intercellular adhesion in pemphigus vulgaris. J. Biol. Chem. 286, 1283–1291 (2011).
Gilhus, N. E. et al. Myasthenia gravis—autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 12, 259–268 (2016).
Lazaridis, K. & Tzartos, S. J. Myasthenia gravis: autoantibody specificities and their role in MG management. Front. Neurol. 11, 596981 (2020).
Granit, V. et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 22, 578–590 (2023). This work reports successful therapy of MG using an mRNA CAR.
Chevet, B. et al. Diagnosing and treating ANCA-associated vasculitis: an updated review for clinical practice. Rheumatology 62, 1787–1803 (2023).
Berglin, E. et al. Anti-neutrophil cytoplasmic antibodies predate symptom onset of ANCA-associated vasculitis. A case–control study. J. Autoimmun. 117, 102579 (2021).
Minopoulou, I. et al. Anti-CD19 CAR T cell therapy induces antibody seroconversion and complete B cell depletion in the bone marrow of a therapy-refractory patient with ANCA-associated vasculitis. Ann. Rheum. Dis. https://doi.org/10.1016/j.ard.2025.01.008 (2025).
Lodka, D. et al. CD19-targeting CAR T cells protect from ANCA-induced acute kidney injury. Ann. Rheum. Dis. 83, 499–507 (2024).
Trivioli, G. et al. Advances in the treatment of ANCA-associated vasculitis. Nat. Rev. Rheumatol. https://doi.org/10.1038/s41584-025-01266-1 (2025).
Bluestone, J. A., Herold, K. & Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010).
Smith, M. J., Simmons, K. M. & Cambier, J. C. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat. Rev. Nephrol. 13, 712–720 (2017).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
Spanier, J. A. et al. Tregs with an MHC class II peptide-specific chimeric antigen receptor prevent autoimmune diabetes in mice. J. Clin. Invest. https://doi.org/10.1172/JCI168601 (2023).
Arnold, D. M. et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann. Intern. Med. 146, 25–33 (2007).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Hawker, K. et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 66, 460–471 (2009).
Hirsch, G. et al. Rituximab, a new treatment for difficult-to-treat chronic erythema multiforme major? Five cases. J. Eur. Acad. Dermatol. Venereol. 30, 1140–1143 (2016).
Joly, P. et al. A single cycle of rituximab for the treatment of severe pemphigus. N. Engl. J. Med. 357, 545–552 (2007).
Colliou, N. et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci. Transl. Med. 5, 175ra130 (2013).
Maloney, D. G., Smith, B. & Rose, A. Rituximab: mechanism of action and resistance. Semin. Oncol. 29, 2–9 (2002).
Horvat, T. Z. et al. The ABCs of immunotherapy for adult patients with B-cell acute lymphoblastic leukemia. Ann. Pharmacother. 52, 268–276 (2018).
Canales-Herrerias, P. et al. High-affinity autoreactive plasma cells disseminate through multiple organs in patients with immune thrombocytopenic purpura. J. Clin. Invest. 132, e153580 (2022).
Cazzaniga, S., Naldi, L. & Borradori, L. Rituximab and risk of infections in patients with pemphigus: answers from a global population-based cohort study. Br. J. Dermatol. 188, 454–455 (2023).
Tony, H. P. et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res. Ther. 13, R75 (2011).
Feldman, R. J. & Ahmed, A. R. Relevance of rituximab therapy in pemphigus vulgaris: analysis of current data and the immunologic basis for its observed responses. Expert. Rev. Clin. Immunol. 7, 529–541 (2011).
Berger, J. R., Malik, V., Lacey, S., Brunetta, P. & Lehane, P. B. Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J. Neurovirol 24, 323–331 (2018).
Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug. Discov. 20, 179–199 (2021).
Danza, A. et al. Prednisone and long-term damage in systemic lupus erythematosus: which is the threshold dose? A pilot study. Lupus 31, 880–884 (2022).
Rekvig, O. P. SLE: a cognitive step forward—a synthesis of rethinking theories, causality, and ignored DNA structures. Front. Immunol. 15, 1393814 (2024).
Burnet, F. M. & Holmes, M. C. The natural history of the NZB/NZW F1 hybrid mouse: a laboratory model of systemic lupus erythematosus. Australas. Ann. Med. 14, 185–191 (1965).
Steinberg, A. D., Roths, J. B., Murphy, E. D., Steinberg, R. T. & Raveche, E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J. Immunol. 125, 871–873 (1980).
Kochenderfer, J. N., Yu, Z., Frasheri, D., Restifo, N. P. & Rosenberg, S. A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–3886 (2010).
Avouac, J. et al. Effects of B Cell depletion by CD19-targeted chimeric antigen receptor T cells in a murine model of systemic sclerosis. Arthritis Rheumatol. 76, 268–278 (2024).
Dai, D. et al. The transcription factor ZEB2 drives the formation of age-associated B cells. Science 383, 413–421 (2024).
Gao, X. et al. Zeb2 drives the formation of CD11c+ atypical B cells to sustain germinal centers that control persistent infection. Sci. Immunol. 9, eadj4748 (2024).
Haga, C. L., Ehrhardt, G. R., Boohaker, R. J., Davis, R. S. & Cooper, M. D. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc. Natl Acad. Sci. USA 104, 9770–9775 (2007).
Zhu, Z., Li, R., Li, H., Zhou, T. & Davis, R. S. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc. Natl Acad. Sci. USA 110, E1282–E1290 (2013).
Dimopoulos, M. A. et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N. Engl. J. Med. 379, 1811–1822 (2018).
Davis, R. S. Fc receptor-like molecules. Annu. Rev. Immunol. 25, 525–560 (2007).
Schett, G. et al. Advancements and challenges in CAR T cell therapy in autoimmune diseases. Nat. Rev. Rheumatol. 20, 531–544 (2024).
Feng, J., Hu, Y.-x., Chang, A. H. & Huang, H. CD19/BCMA CAR-T cell therapy for refractory systemic lupus erythematosus—safety and preliminary efficacy data from a phase I clinical study. Blood 142, 4835 (2023).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022). This pioneering work assesses tolerability and efficacy of CD19 CAR T cells in refractory patients with SLE and provided the proof of concept for the expansion of CAR T cell therapies to autoimmune indications.
Zhang, W. et al. Treatment of systemic lupus erythematosus using BCMA–CD19 compound CAR. Stem Cell Rev. Rep. 17, 2120–2123 (2021). This work is the first use of dual targeting CAR T cell therapy in AIDs.
Fischbach, F. et al. CD19-targeted chimeric antigen receptor T cell therapy in two patients with multiple sclerosis. Med 5, 550–558.e2 (2024). This work is the first report of CAR T cell therapy in MS.
Richter, J. et al. CD19-directed CAR T cell therapy in 4 patients with refractory multiple sclerosis. Blood 144, 2073 (2024).
Krickau, T. et al. CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. Lancet 403, 1627–1630 (2024).
Auth, J. et al. CD19-targeting CAR T-cell therapy in patients with diffuse systemic sclerosis: a case series. Lancet Rheumatol. https://doi.org/10.1016/S2665-9913(24)00282-0 (2024).
Sheng, L. et al. Concurrent remission of lymphoma and Sjogren’s disease following anti-CD19 chimeric antigen receptor-T cell therapy for diffuse large B-cell lymphoma: a case report. Front. Immunol. 14, 1298815 (2023).
Pecher, A. C. et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA 329, 2154–2162 (2023).
Taubmann, J. et al. Rescue therapy of antisynthetase syndrome with CD19-targeted CAR-T cells after failure of several B-cell depleting antibodies. Rheumatology 63, e12–e14 (2024).
Haghikia, A. et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 22, 1104–1105 (2023).
Motte, J. et al. Treatment of concomitant myasthenia gravis and Lambert–Eaton myasthenic syndrome with autologous CD19-targeted CAR T cells. Neuron 112, 1757–1763.e2 (2024).
Nicolai, R. et al. Autologous CD19-targeting CAR T cells in a patient with refractory juvenile dermatomyositis. Arthritis Rheumatol. 76, 1560–1565 (2024).
Volkov, J. et al. Case study of CD19 CAR T therapy in a subject with immune-mediate necrotizing myopathy treated in the RESET-Myositis phase I/II trial. Mol. Ther. 32, 3821–3828 (2024).
Tur, C. et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann. Rheum. Dis. 84, 106–114 (2025). This study demonstrates that CD19-CAR T cell therapy induces profound B cell depletion in secondary lymphoid tissues of patients with SLE.
Wilhelm, A. et al. Selective CAR T cell-mediated B cell depletion suppresses IFN signature in SLE. JCI Insight 9, 179433 (2024).
Hagen, M. M. et al. Safety and long-term efficacy of CD19-CAR T-cell therapy in 30 patients with autoimmune disease [abstract]. Arthritis Rheumatol. 76, 1749 (2024).
Hagen, M. et al. Local immune effector cell-associated toxicity syndrome in CAR T-cell treated patients with autoimmune disease: an observational study. Lancet Rheumatol. 7, e424–e433 (2025).
He, X. et al. Treatment of two pediatric patients with refractory systemic lupus erythematosus using CD19-targeted CAR T-cells. Autoimmun. Rev. 24, 103692 (2025).
Gray, G. I. et al. The evolving T cell receptor recognition code: the rules are more like guidelines. Immunol. Rev. 329, e13439 (2025).
Alarcon, B. & Schamel, W. W. Allosteric changes underlie the outside-in transmission of activatory signals in the TCR. Immunol. Rev. 329, e13438 (2025).
Jayaraman, J. et al. CAR-T design: elements and their synergistic function. EBioMedicine 58, 102931 (2020).
Baker, D. J., Arany, Z., Baur, J. A., Epstein, J. A. & June, C. H. CAR T therapy beyond cancer: the evolution of a living drug. Nature 619, 707–715 (2023).
Esensten, J. H., Helou, Y. A., Chopra, G., Weiss, A. & Bluestone, J. A. CD28 costimulation: from mechanism to therapy. Immunity 44, 973–988 (2016).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Balagopalan, L. et al. Generation of antitumor chimeric antigen receptors incorporating T cell signaling motifs. Sci. Signal. 17, eadp8569 (2024).
Castellanos-Rueda, R. et al. speedingCARs: accelerating the engineering of CAR T cells by signaling domain shuffling and single-cell sequencing. Nat. Commun. 13, 6555 (2022).
Goodman, D. B. et al. Pooled screening of CAR T cells identifies diverse immune signaling domains for next-generation immunotherapies. Sci. Transl. Med. 14, eabm1463 (2022).
Rios, X. et al. Refining chimeric antigen receptors via barcoded protein domain combination pooled screening. Mol. Ther. 31, 3210–3224 (2023).
Frigault, M. J. et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 3, 356–367 (2015).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Wu, L. et al. CD28-CAR-T cell activation through FYN kinase signaling rather than LCK enhances therapeutic performance. Cell Rep. Med. 4, 100917 (2023).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
van Oers, N. S., Killeen, N. & Weiss, A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCRζ in murine thymocytes and lymph node T cells. Immunity 1, 675–685 (1994).
van Oers, N. S. et al. Constitutive tyrosine phosphorylation of the T-cell receptor (TCR) ζ subunit: regulation of TCR-associated protein tyrosine kinase activity by TCRζ. Mol. Cell Biol. 13, 5771–5780 (1993).
Ajina, A. & Maher, J. Strategies to address chimeric antigen receptor tonic signaling. Mol. Cancer Ther. 17, 1795–1815 (2018).
Minguet, S., Maus, M. V. & Schamel, W. W. From TCR fundamental research to innovative chimeric antigen receptor design. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-024-01093-7 (2024).
Baeuerle, P. A. et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 10, 2087 (2019).
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Wang, X. et al. Allogeneic CD19-targeting T cells for treatment-refractory systemic lupus erythematosus: a phase 1 trial. Nat. Med. https://doi.org/10.1038/s41591-025-03899-x (2025). This work shows that allogeneic CAR T cell therapy can be safe and efficacious in AIDs.
Kvalvaag, A. & Dustin, M. L. Clathrin controls bidirectional communication between T cells and antigen presenting cells. Bioessays 46, e2300230 (2024).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Lungova, K. & Putman, M. Barriers to CAR T-cell therapy in rheumatology. Lancet Rheumatol. 7, e212–e216 (2025).
Borgert, R. Improving outcomes and mitigating costs associated with CAR T-cell therapy. Am. J. Manag. Care 27, S253–S261 (2021).
Mansoori, S., Noei, A., Maali, A., Seyed-Motahari, S. S. & Sharifzadeh, Z. Recent updates on allogeneic CAR-T cells in hematological malignancies. Cancer Cell Int. 24, 304 (2024).
Caldwell, K. J., Gottschalk, S. & Talleur, A. C. Allogeneic CAR cell therapy—more than a pipe dream. Front. Immunol. 11, 618427 (2020).
Moradi, V., Omidkhoda, A. & Ahmadbeigi, N. The paths and challenges of “off-the-shelf” CAR-T cell therapy: an overview of clinical trials. Biomed. Pharmacother. 169, 115888 (2023). This review surveys advanced allogeneic strategies in clinical development.
Peng, L., Sferruzza, G., Yang, L., Zhou, L. & Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol. Immunol. 21, 1089–1108 (2024).
Acharya, S. et al. CD28 costimulation augments CAR signaling in NK cells via the LCK/CD3ζ/ZAP70 signaling axis. Cancer Discov. 14, 1879–1900 (2024).
Page, A., Chuvin, N., Valladeau-Guilemond, J. & Depil, S. Development of NK cell-based cancer immunotherapies through receptor engineering. Cell Mol. Immunol. 21, 315–331 (2024).
Jorgensen, L. V., Christensen, E. B., Barnkob, M. B. & Barington, T. The clinical landscape of CAR NK cells. Exp. Hematol. Oncol. 14, 46 (2025).
Yu, J. et al. CAR immunotherapy in autoimmune diseases: promises and challenges. Front. Immunol. 15, 1461102 (2024).
Zhang, Q. et al. Therapeutic potential of natural killer cells in neuroimmunological diseases. Biomed. Pharmacother. 173, 116371 (2024).
Bialy, S. & Bogunia-Kubik, K. Uncovering the mysteries of human γδ T cells: from origins to novel therapeutics. Front. Immunol. 16, 1543454 (2025).
Skuljec, J. et al. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front. Immunol. 8, 1125 (2017). This work demonstrates that CAR-engineered Treg cells effectively suppress allergic airway inflammation in a mouse model of asthma.
Zhang, Q. et al. Chimeric antigen receptor (CAR) Treg: a promising approach to inducing immunological tolerance. Front. Immunol. 9, 2359 (2018).
Selck, C. & Dominguez-Villar, M. Antigen-specific regulatory T cell therapy in autoimmune diseases and transplantation. Front. Immunol. 12, 661875 (2021).
Yeh, W. I. et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing de novo autoreactive T-cell receptors in type 1 diabetes. Front. Immunol. 8, 1313 (2017).
Arjomandnejad, M. et al. Modulating immune responses to AAV by expanded polyclonal T-regs and capsid specific chimeric antigen receptor T-regulatory cells. Mol. Ther. Methods Clin. Dev. 23, 490–506 (2021).
Proics, E. et al. Preclinical assessment of antigen-specific chimeric antigen receptor regulatory T cells for use in solid organ transplantation. Gene Ther. 30, 309–322 (2023).
Kim, Y. C. et al. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. J. Autoimmun. 92, 77–86 (2018). This work demonstrates that engineered MBP-specific human Treg cells can suppress autoimmunity in an MS model.
Lamarthee, B. et al. Transient mTOR inhibition rescues 4-1BB CAR-Tregs from tonic signal-induced dysfunction. Nat. Commun. 12, 6446 (2021).
Requejo Cier, C. J., Valentini, N. & Lamarche, C. Unlocking the potential of Tregs: innovations in CAR technology. Front. Mol. Biosci. 10, 1267762 (2023).
Khawar, M. B., Afzal, A., Si, Y. & Sun, H. Steering the course of CAR T cell therapy with lipid nanoparticles. J. Nanobiotechnology 22, 380 (2024).
Agarwalla, P. et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat. Biotechnol. 40, 1250–1258 (2022).
Short, L., Holt, R. A., Cullis, P. R. & Evgin, L. Direct in vivo CAR T cell engineering. Trends Pharmacol. Sci. 45, 406–418 (2024). This study presents a comprehensive overview of emerging strategies to generate CAR T cells in vivo.
Michels, A., Ho, N. & Buchholz, C. J. Precision medicine: in vivo CAR therapy as a showcase for receptor-targeted vector platforms. Mol. Ther. 30, 2401–2415 (2022).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020). This work is an elegant demonstration of in vivo CAR construction using targeted LNPs.
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). This work provides a preclinical proof of concept for in vivo generation of CAR T cells, using T cell-targeted LNPs, to treat cardiac fibrosis.
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug. Discov. 20, 817–838 (2021).
Kon, E., Ad-El, N., Hazan-Halevy, I., Stotsky-Oterin, L. & Peer, D. Targeting cancer with mRNA–lipid nanoparticles: key considerations and future prospects. Nat. Rev. Clin. Oncol. 20, 739–754 (2023).
Meng, S. et al. In vivo engineered CAR-T cell therapy: lessons built from COVID-19 mRNA vaccines. Int. J. Mol. Sci. https://doi.org/10.3390/ijms26073119 (2025).
Metzloff, A. E. et al. Antigen presenting cell mimetic lipid nanoparticles for rapid mRNA CAR T cell cancer immunotherapy. Adv. Mater. 36, e2313226 (2024).
Odendahl, M. et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165, 5970–5979 (2000).
Anolik, J. H. et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 50, 3580–3590 (2004).
Reijm, S. et al. Autoreactive B cells in rheumatoid arthritis include mainly activated CXCR3+ memory B cells and plasmablasts. JCI Insight 8, e172006 (2023).
Wang, Y. et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naive repertoire consistent with defects in B-cell tolerance. Sci. Rep. 9, 19995 (2019).
Eggers, E. L. et al. Clonal relationships of CSF B cells in treatment-naive multiple sclerosis patients. JCI Insight 2, e92724 (2017).
Palanichamy, A. et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci. Transl. Med. 6, 248ra106 (2014).
Hammers, C. M. et al. Persistence of anti-desmoglein 3 IgG+ B-cell clones in pemphigus patients over years. J. Invest. Dermatol. 135, 742–749 (2015).
Pollmann, R. et al. Identification of autoreactive B cell subpopulations in peripheral blood of autoimmune patients with pemphigus vulgaris. Front. Immunol. 10, 1375 (2019).
El-Mokhtar, M. A. et al. Altered regulatory B cell subsets in children with type 1 diabetes mellitus. J. Immunol. Res. 2020, 8935694 (2020).
Stensland, Z. C. et al. Identification of an anergic BND cell-derived activated B cell population (BND2) in young-onset type 1 diabetes patients. J. Exp. Med. 220, e20221604 (2023).
Kohler, S. et al. Disturbed B cell subpopulations and increased plasma cells in myasthenia gravis patients. J. Neuroimmunol. 264, 114–119 (2013).
Stathopoulos, P., Kumar, A., Nowak, R. J. & O’Connor, K. C. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2, e94263 (2017).
Elinav, E., Adam, N., Waks, T. & Eshhar, Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology 136, 1721–1731 (2009).
Fransson, M. et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflamm. 9, 112 (2012).
Oh, S. et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat. Biotechnol. 41, 1229–1238 (2023).
Reincke, S. M. et al. Chimeric autoantibody receptor T cells deplete NMDA receptor-specific B cells. Cell 186, 5084–5097.e18 (2023).
Gardner, R. et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 127, 2406–2410 (2016).
Westin, J. R. et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N. Engl. J. Med. 389, 148–157 (2023).
Martin, T. et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 41, 1265–1274 (2023).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Frank, M. J. et al. CD22-directed CAR T-cell therapy for large B-cell lymphomas progressing after CD19-directed CAR T-cell therapy: a dose-finding phase 1 study. Lancet 404, 353–363 (2024).
Lamble, A. J. et al. Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv. 7, 575–585 (2023).
Lee, H. et al. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat. Med. 29, 2295–2306 (2023).
Orlando, E. J. et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 24, 1504–1506 (2018).
Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 5, 1282–1295 (2015).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Ghorashian, S. et al. CD19/CD22 targeting with cotransduced CAR T cells to prevent antigen-negative relapse after CAR T-cell therapy for B-cell ALL. Blood 143, 118–123 (2024).
Schultz, L. M. et al. CD22 CAR T cells demonstrate high response rates and safety in pediatric and adult B-ALL: phase 1b results. Leukemia https://doi.org/10.1038/s41375-024-02220-y (2024).
Li, P. et al. C-CAR066, a novel fully human anti-CD20 CAR-T therapy for relapsed or refractory large B-cell lymphoma after failure of anti-CD19 CAR-T therapy: a phase I clinical study. Am. J. Hematol. 99, 2306–2312 (2024).
Larson, S. M. et al. CD19/CD20 bispecific chimeric antigen receptor (CAR) in naive/memory T cells for the treatment of relapsed or refractory non-Hodgkin lymphoma. Cancer Discov. 13, 580–597 (2023).
Shah, N. N. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med. 26, 1569–1575 (2020).
Hines, M. R. et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transpl. Cell Ther. 29, 438.e1–438.e16 (2023).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Maus, M. V. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8, e001511 (2020).
Gust, J. et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Gofshteyn, J. S. et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann. Neurol. 84, 537–546 (2018).
Gust, J., Taraseviciute, A. & Turtle, C. J. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs 32, 1091–1101 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e17 (2020).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Diorio, C. et al. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities. Blood Adv. 6, 3398–3403 (2022).
Kattamuri, L. et al. Safety and efficacy of CAR-T cell therapy in patients with autoimmune diseases: a systematic review. Rheumatol. Int. 45, 18 (2025).
Acknowledgements
The authors acknowledge information gathered by J. J. Knox, University of Pennsylvania, that was used in Table 2. A.B. acknowledges funding from the European Research Council (ERC) (08930382000), Boaz and Varda Dotan, and the Israeli Ministry of Health (00370000015). R.S.D. was supported in part by the Leukaemia and Lymphoma Society (LLS). M.S. is supported by the Bettencourt-Schueller Foundation, the INSERM, Ecole de l’INSERM Bettencourt-Schueller, the FOREUM foundation and the Arthritis Pierre Coubertin foundation. S.G. is supported by the American Lebanese Syrian Associated Charities (ALSAC). A.T. is supported by 1K08CA279927-01A1 from the National Institutes of Health (NIH). M.R. receives research support from the Alliance for Lupus Research, LLS and the Oxnard Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article and are listed alphabetically.
Corresponding author
Ethics declarations
Competing interests
M.S. is a consultant for Abbvie, Amgen, AstraZeneca, Biogen, BMS, Fresenius, Galapagos, GSK, Innate Pharma, Nordic Pharma, Novartis, Roche and Sandoz. S.G. is a member of the Data Safety Monitoring Board (DSMB) of Immatics, serves on the Scientific Advisory Board of Be Biopharma, served as a consultant for CARGO Therapeutics within the last 12 months, and has patents and patent applications in the fields of T cell and/or gene therapy for cancer. M.R. has consulted for Bain Capital, Guidepoint Global LLC and NVP Associates. The other authors declare no additional competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Laura Evgin, Christopher Jewell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Avouac, J., Barzel, A., Caiati, D. et al. Roads and detours for CAR T cell therapy in autoimmune diseases. Nat Rev Drug Discov (2026). https://doi.org/10.1038/s41573-025-01349-4
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41573-025-01349-4