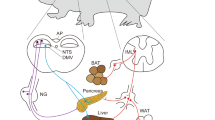
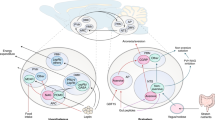
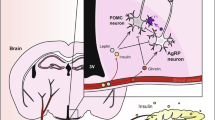
Abstract
The autonomic nervous system is a crucial mediator between the central nervous system and peripheral tissues and is essential for maintaining homeostasis. In this Review, we discuss the bidirectional communication between the autonomic nervous system and metabolic tissues in humans, focusing on the coordination of systemic glucose and lipid metabolism through autonomic signalling across changing physiological states. We also discuss the crosstalk between autonomic and immune pathways and its relevance for metabolic control. An overview of current methodologies to assess autonomic function in humans shows that quantifying organ-specific autonomic outflows remains challenging. Chronic disturbances in autonomic regulation are increasingly recognized as contributors to metabolic diseases such as obesity and type 2 diabetes mellitus. Hence, emerging therapeutic strategies targeting autonomic function could offer promising opportunities to improve metabolic health. Progress will depend on the development of tools to selectively assess autonomic input to individual metabolic organs. Addressing high inter-individual variability and capturing the temporal dynamics of organ-specific autonomic regulation will be essential for advancing mechanistic insights, ultimately enabling clinical translation.
Key points
-
The autonomic nervous system regulates glucose metabolism via coordinated brain–periphery signalling, regulating insulin secretion, hepatic glucose production, adipose lipolysis and skeletal muscle glucose uptake among other functions.
-
Both sympathetic and parasympathetic branches of the autonomic nervous system contribute to glucose homeostasis in a context-dependent manner, shaped by feeding status, stress, circadian rhythm and immune interactions.
-
Organ-specific autonomic inputs are challenging to assess; current measures (for example, heart rate variability) provide only partial insights into metabolic autonomic regulation.
-
Chronic autonomic imbalance, especially increased sympathetic tone, is implicated in the development of obesity, insulin resistance and type 2 diabetes mellitus.
-
Emerging interventions, including lifestyle changes, pharmacotherapy and device-based neuromodulation, offer promising approaches to restore autonomic balance and improve metabolic outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Langley, J. N. The Autonomic Nervous System (W. Heffer & Sons, 1921).
Roche, F. et al. Anatomy and physiology of the autonomic nervous system: implication on the choice of diagnostic/monitoring tools in 2023. Rev. Neurologique 180, 42–52 (2024).
Teff, K. L. Visceral nerves: vagal and sympathetic innervation. J. Parenter. Enter. Nutr. 32, 569–571 (2008).
Levy, M. N. Sympathetic-parasympathetic interactions in the heart. Circulation Res. 29, 437–445 (1971).
Uijtdehaage, S. H. J. & Thayer, J. F. Accentuated antagonism in the control of human heart rate. Clin. Autonomic Res. 10, 107–110 (2000).
Thayer, J. F. & Sternberg, E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann. N. Y. Acad. Sci. 1088, 361–372 (2006).
Beissner, F., Meissner, K., Bar, K.-J. & Napadow, V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33, 10503–10511 (2013).
Heni, M. The insulin resistant brain: impact on whole-body metabolism and body fat distribution. Diabetologia 67, 1181–1191 (2024).
Kronsteiner, B. et al. Characterization, number, and spatial organization of nerve fibers in the human cervical vagus nerve and its superior cardiac branch. Brain Stimulation 17, 510–524 (2024).
Lin, E. E., Scott-Solomon, E. & Kuruvilla, R. Peripheral innervation in the regulation of glucose homeostasis. Trends Neurosci. 44, 189–202 (2021).
Stenvers, D. J., Scheer, F. A. J. L., Schrauwen, P., La Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Shen, T., Tang, X., Pan, Y., He, H. & Hu, K. Effect of vagus nerve stimulation on metabolism: a systematic review and meta-analysis. Int. J. Obes. 49, 2383–2394 (2025).
Koutra, E. et al. Unravelling the effect of renal denervation on glucose homeostasis: more questions than answers? Acta Diabetol. 61, 267–280 (2023).
Carnagarin, R. et al. Effects of sympathetic modulation in metabolic disease. Ann. N. Y. Acad. Sci. 1454, 80–89 (2019).
Coopmans, C. et al. Both prediabetes and type 2 diabetes are associated with lower heart rate variability: the Maastricht study. Diabetes Care 43, 1126–1133 (2020).
Jarczok, Koenig, J., Schuster, A. K., Thayer, J. F. & Fischer, J. E. Nighttime heart rate variability, overnight urinary norepinephrine, and glycemic status in apparently healthy human adults. Int. J. Cardiol. 168, 3025–3026 (2013).
Schuster, A. K., Fischer, J. E., Thayer, J. F., Mauss, D. & Jarczok, M. N. Decreased heart rate variability correlates to increased cardiovascular risk. Int. J. Cardiol. 203, 728–730 (2016).
Jarczok, M. N. et al. Heart rate variability in the prediction of mortality: a systematic review and meta-analysis of healthy and patient populations. Neurosci. Biobehav. Rev. 143, 104907 (2022).
Thayer, J. F., Mather, M. & Koenig, J. Stress and aging: a neurovisceral integration perspective. Psychophysiology 58, e13804 (2021).
Schmalenberger, K. M. et al. A systematic review and meta-analysis of within-person changes in cardiac vagal activity across the menstrual cycle: implications for female health and future studies. J. Clin. Med. 8, 1946 (2019).
Vallbo, ÅB., Hagbarth, K.-E. & Wallin, B. G. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J. Appl. Physiol. 96, 1262–1269 (2004).
Egan, B. M. Insulin resistance and the sympathetic nervous system. Curr. Sci. Inc. 5, 247–254 (2003).
Shoemaker, J. K., Klassen, S. A., Badrov, M. B. & Fadel, P. J. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J. Neurophysiol. 119, 1731–1744 (2018).
Seravalle, G. & Grassi, G. Sympathetic nervous system and hypertension: new evidences. Autonomic Neurosci. 238, 102954 (2022).
Laborde, S., Mosley, E. & Thayer, J. F. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 8, 213 (2017).
Buijs, R. M. & Kreier, F. The metabolic syndrome: a brain disease? J. Neuroendocrinol. 18, 715–716 (2006).
Yu, T. Y. & Lee, M. Autonomic dysfunction, diabetes and metabolic syndrome. J. Diabetes Invest. 12, 2108–2111 (2021).
Hyun, U. & Sohn, J.-W. Autonomic control of energy balance and glucose homeostasis. Exp. Mol. Med. 54, 370–376 (2022).
Waise, T. M. Z., Dranse, H. J. & Lam, T. K. T. The metabolic role of vagal afferent innervation. Nat. Rev. Gastroenterol. Hepatol. 15, 625–636 (2018).
Imai, J. & Katagiri, H. Regulation of systemic metabolism by the autonomic nervous system consisting of afferent and efferent innervation. Int. Immunol. 34, 67–79 (2022).
Thorens, B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes. Metab. 13, 82–88 (2011).
Thorens, B. Neuronal glucose sensing mechanisms and circuits in the control of insulin and glucagon secretion. Physiological Rev. 104, 1461–1486 (2024).
Sohn, J.-W., Elmquist, J. K. & Williams, K. W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512 (2013).
Sohn, J.-W. Network of hypothalamic neurons that control appetite. BMB Rep. 48, 229–233 (2015).
Van Baak, M. A. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol. Behav. 94, 178–186 (2008).
Matheson, P. J., Wilson, M. A. & Garrison, R. N. Regulation of intestinal blood flow. J. Surgical Res. 93, 182–196 (2000).
Fagius, J. Sympathetic nerve activity in metabolic control — some basic concepts. Acta Physiologica Scandinavica 177, 337–343 (2003).
Fugmann, A., Millgård, J., Sarabi, M., Berne, C. & Lind, L. Central and peripheral haemodynamic effects of hyperglycaemia, hyperinsulinaemia, hyperlipidaemia or a mixed meal. Clin. Sci. 105, 715–721 (2003).
Waaler, B. A. & Eriksen, M. Post-prandial cardiovascular responses in man after ingestion of carbohydrate, protein or fat. Acta Physiologica Scandinavica 146, 321–327 (1992).
Fagius, J. & Berne, C. Increase in muscle nerve sympathetic activity in humans after food intake. Clin. Sci. 86, 159–167 (1994).
Brooks, G. A. & Mercier, J. Balance of carbohydrate and lipid utilization during exercise: the ‘crossover’ concept. J. Appl. Physiol. 76, 2253–2261 (1994).
Kjaer, M., Engfred, K., Fernandes, A., Secher, N. H. & Galbo, H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. Am. J. Physiol. Endocrinol. Metab. 265, E275–E283 (1993).
Hoffman, R. P. Sympathetic mechanisms of hypoglycemic counterregulation. Curr Diabetes Rev. 3, 185–193 (2007).
Pongratz, G. & Straub, R. H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 16, 504 (2014).
Hallschmid, M. Intranasal insulin. J. Neuroendocrinol. 33, e12934 (2021).
Heni, M. et al. Insulin action in the hypothalamus increases second-phase insulin secretion in humans. Neuroendocrinology 110, 929–937 (2020).
Kullmann, S. et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 8, 524–534 (2020).
Borgmann, D. & Fenselau, H. Vagal pathways for systemic regulation of glucose metabolism. Semin. Cell Dev. Biol. 156, 244–252 (2024).
Hampton, R. F., Jimenez-Gonzalez, M. & Stanley, S. A. Unravelling innervation of pancreatic islets. Diabetologia 65, 1069–1084 (2022).
Kalsbeek, A. et al. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann. N. Y. Acad. Sci. 1212, 114–129 (2010).
Heni, M. et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 63, 4083–4088 (2014).
Kullmann, S. et al. Dose-dependent effects of intranasal insulin on resting-state brain activity. J. Clin. Endocrinol. Metab. 103, 253–262 (2018).
Ruud, J., Steculorum, S. M. & Brüning, J. C. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat. Commun. 8, 15259 (2017).
Heni, M. et al. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66, 1797–1806 (2017).
Heni, M. et al. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 55, 1773–1782 (2012).
Dash, S., Xiao, C., Morgantini, C., Koulajian, K. & Lewis, G. F. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64, 766–774 (2015).
Kishore, P. et al. Activation of KATP channels suppresses glucose production in humans. J. Clin. Invest. 121, 4916–4920 (2011).
Gancheva, S. et al. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes 64, 1966–1975 (2015).
Plomgaard, P. et al. Nasal insulin administration does not affect hepatic glucose production at systemic fasting insulin levels. Diabetes Obes. Metab. 21, 993–1000 (2019).
Hallschmid, M. et al. Intranasal insulin reduces body fat in men but not in women. Diabetes 53, 3024–3029 (2004).
Hummel, J. et al. Brain insulin action on peripheral insulin sensitivity in women depends on menstrual cycle phase. Nat. Metab. 5, 1475–1482 (2023).
Hummel, J., Kullmann, S., Wagner, R. & Heni, M. Glycaemic fluctuations across the menstrual cycle: possible effect of the brain. Lancet Diabetes Endocrinol. 11, 883–884 (2023).
Porte, D. & Williams, R. H. Inhibition of insulin release by norepinephrine in man. Science 152, 1248–1250 (1966).
Ahrén, B. Autonomic regulation of islet hormone secretion — implications for health and disease. Diabetologia 43, 393–410 (2000).
Porte, D. Beta adrenergic stimulation of insulin release in man. Diabetes 16, 150–155 (1967).
Porte, D. A receptor mechanism for the inhibition of insulin release by epinephrine in man. J. Clin. Invest. 46, 86–94 (1967).
Campfield, L. A., Smith, F. J. & Eskinazi, R. E. Glucose responsiveness and acetylcholine sensitivity of pancreatic beta-cells after vagotomy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 246, R985–R993 (1984).
Campfield, L. A. & Blocker, D. C. Simulation of the autonomic neural control of insulin secretion. Comput. Biol. Med. 9, 191–203 (1979).
Adablah, J. E., Vinson, R., Roper, M. G. & Bertram, R. Synchronization of pancreatic islets by periodic or non-periodic muscarinic agonist pulse trains. PLoS ONE 14, e0211832 (2019).
Gautam, D. et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 3, 449–461 (2006).
Azua, I. R. D., Gautam, D., Guettier, J.-M. & Wess, J. Novel insights into the function of β-cell M3 muscarinic acetylcholine receptors: therapeutic implications. Trends Endocrinol. Metab. 22, 74–80 (2011).
Begg, D. P. & Woods, S. C. Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv. Physiol. Educ. 37, 53–60 (2013).
Thorens, B. GLUT2 in pancreatic and extra-pancreatic gluco-detection. Mol. Membr. Biol. 18, 265–273 (2001).
Gromada, J. et al. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J. Physiol. 518, 745–759 (1999).
Wagner, R. et al. Nonsuppressed glucagon after glucose challenge as a potential predictor for glucose tolerance. Diabetes 66, 1373–1379 (2017).
Wagner, R. et al. Postprandial dynamics of proglucagon cleavage products and their relation to metabolic health. Front. Endocrinol. 13, 892677 (2022).
Faber, C. L., Deem, J. D., Campos, C. A., Taborsky, G. J. & Morton, G. J. CNS control of the endocrine pancreas. Diabetologia 63, 2086–2094 (2020).
Taborsky, G. J. & Mundinger, T. O. Minireview: the role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology 153, 1055–1062 (2012).
Ojha, A., Ojha, U., Mohammed, R., Chandrashekar, A. & Ojha, H. Current perspective on the role of insulin and glucagon in the pathogenesis and treatment of type 2 diabetes mellitus. Clin. Pharmacol. 11, 57–65 (2019).
Havel, P. J., Veith, R. C., Dunning, B. E. & Taborsky, G. J. Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog. Evidence for stress-specific and regionally selective activation of the sympathetic nervous system. J. Clin. Invest. 82, 1538–1545 (1988).
Thorens, B. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab. 16, 87–95 (2014).
Perseghin, G. et al. Regulation of glucose homeostasis in humans with denervated livers. J. Clin. Invest. 100, 931–941 (1997).
Verberne, A. J. M., Korim, W. S., Sabetghadam, A. & Llewellyn-Smith, I. J. Adrenaline: insights into its metabolic roles in hypoglycaemia and diabetes. Br. J. Pharmacol. 173, 1425–1437 (2016).
Makhmutova, M. et al. Pancreatic β-cells communicate with vagal sensory neurons. Gastroenterology 160, 875–888.e11 (2021).
Hauge-Evans, A. C. et al. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58, 403–411 (2009).
DeFronzo, R. A. & Ferrannini, E. Regulation of hepatic glucose metabolism in humans. Diabetes Metab. Rev. 3, 415–459 (1987).
Yi, C.-X., La Fleur, S. E., Fliers, E. & Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta 1802, 416–431 (2010).
Shimazu, T. & Ogasawara, S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am. J. Physiol. 228, 1787–1793 (1975).
Burcelin, R. et al. Impaired glucose homeostasis in mice lacking the α1b-adrenergic receptor subtype. J. Biol. Chem. 279, 1108–1115 (2004).
Mirzadeh, Z., Faber, C. L. & Schwartz, M. W. Central nervous system control of glucose homeostasis: a therapeutic target for type 2 diabetes? Annu. Rev. Pharmacol. Toxicol. 62, 55–84 (2022).
Chu, C. A. et al. The direct effects of catecholamines on hepatic glucose production occur via α1 - and β2-receptors in the dog. Am. J. Physiol. Endocrinol. Metab. 279, E463–E473 (2000).
Shimazu, T. Regulation of glycogen metabolism in liver by the autonomic nervous system. V. Activation of glycogen synthetase by vagal stimulation. Biochim. Biophys. Acta 252, 28–38 (1971).
Shimazu, T. Glycogen synthetase activity in liver: regulation by the autonomic nerves. Science 156, 1256–1257 (1967).
Matsuhisa, M. et al. Important role of the hepatic vagus nerve in glucose uptake and production by the liver. Metabolism 49, 11–16 (2000).
Vatamaniuk, M. Z., Horyn, O. V., Vatamaniuk, O. K. & Doliba, N. M. Acetylcholine affects rat liver metabolism via type 3 muscarinic receptors in hepatocytes. Life Sci. 72, 1871–1882 (2003).
Moore, M. & Cherrington, A. Regulation of net hepatic glucose uptake: interaction of neural and pancreatic mechanisms. Reprod. Nutr. Dev. 36, 399–406 (1996).
Ahrén, B. The neuro-incretin concept. Regulatory Pept. 194–195, 3–5 (2014).
Moore, M. C., Coate, K. C., Winnick, J. J., An, Z. & Cherrington, A. D. Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 3, 286–294 (2012).
Mizuno, K. & Ueno, Y. Autonomic nervous system and the liver. Hepatol. Res. 47, 160–165 (2017).
Lautt, W. W. et al. Hepatic parasympathetic (HISS) control of insulin sensitivity determined by feeding and fasting. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G29–G36 (2001).
Saad, A. et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61, 2691–2700 (2012).
Flaa, A., Aksnes, T. A., Kjeldsen, S. E., Eide, I. & Rostrup, M. Increased sympathetic reactivity may predict insulin resistance: an 18-year follow-up study. Metabolism 57, 1422–1427 (2008).
Masuo, K. Sympathetic nerve hyperactivity precedes hyperinsulinemia and blood pressure elevation in a young, nonobese Japanese population. Am. J. Hypertension 10, 77–83 (1997).
Gamboa, A. et al. Autonomic blockade improves insulin sensitivity in obese subjects. Hypertension 64, 867–874 (2014).
Bruinstroop, E., Fliers, E. & Kalsbeek, A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract. Res. Clin. Endocrinol. Metab. 28, 673–684 (2014).
Metz, M. et al. Leptin increases hepatic triglyceride export via a vagal mechanism in humans. Cell Metab. 34, 1719–1731 (2022).
Giovanini, L., Wanionok, N., Perello, M. & Cornejo, M. P. Brain-acting hepatokines: its impact on energy balance and metabolism. Front. Neurosci. 19, 1589110 (2025).
Miao, X. et al. Hepatokines: unveiling the molecular and cellular mechanisms connecting hepatic tissue to insulin resistance and inflammation. Acta Diabetol. 61, 1339–1361 (2024).
Owen, B. M. et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20, 670–677 (2014).
Rose, J. P. et al. FGF21 reverses MASH through coordinated actions on the CNS and liver. Cell Metab. 37, 1515–1529 (2025).
Colle, I., Van Vlierberghe, H., Troisi, R. & De Hemptinne, B. Transplanted liver: consequences of denervation for liver functions. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 280, 924–931 (2004).
Rameshi, Y. et al. Peri-liver transplant hyperglycemia: mechanisms, associated factors, consequences, and management — a systematic review. Endocrinol. Diabetes Metab. 8, e70107 (2025).
Gabrielli, F. et al. Side effects of immunosuppressant drugs after liver transplant. Pharmaceuticals 18, 342 (2025).
Burns, T. W. & Hales, C. N. Regulation of lipolysis in isolated human adipose tissue cells. Lancet 287, 796–798 (1966).
Burns, T. W. & Langley, P. E. Lipolysis by human adipose tissue: the role of cyclic 3’,5’-adenosine monophosphate and adrenergic receptor sites. J. Lab. Clin. Med. 75, 983–997 (1970).
Nielsen, S. L., Bitsch, V., Larsen, O. A., Lassen, N. A. & Quaade, F. Blood flow through human adipose tissue during lipolysis. Scand. J. Clin. Lab. Investig. 22, 124–130 (1968).
Leboeuf, B., Flinn, R. B. & Cahill, G. F. Effect of epinephrine on glucose uptake and glycerol release by adipose tissue in vitro. Exp. Biol. Med. 102, 527–529 (1959).
Fessler, A., Beck, J. C. & Rubinstein, D. Factors affecting lipid synthesis in human adipose tissue in vitro. Metabolism 16, 438–444 (1967).
Efendić, S. & Ostman, J. Catecholamines and metabolism of human adipose tissue. V. Studies on the incorporation of glucose-1-14C into lipids and the re-esterification of FFA by human omental tissue in vitro. Acta Med. Scand. 187, 493–502 (1970).
Östman, J., Arner, P., Kimura, H., Wahrenberg, H. & Engfeldt, P. Influence of fasting on lipolytic response to adrenergic agonists and on adrenergic receptors in subcutaneous adipocytes. Eur. J. Clin. Investig. 14, 383–391 (1984).
Arner, P., Engfeldt, P. & Östman, J. Relationship between lipolysis, cyclic AMP, and fat-cell size in human adipose tissue during fasting and in diabetes mellitus. Metabolism 28, 198–209 (1979).
Burns, T. W., Boyer, P. A., Terry, B. E., Langley, P. E. & Robison, G. A. The effect of fasting on the adrenergic receptor activity of human adipocytes. J. Lab. Clin. Med. 94, 387–394 (1979).
Bülow, J. Human adipose tissue blood flow during prolonged exercise, III. Effect of F-adrenergic blockade, nicotinic acid and glucose infusion. Scand. J. Clin. Lab. Investig. 41, 415–424 (1981).
Ahlborg, G., Felig, P., Hagenfeldt, L., Hendler, R. & Wahren, J. Substrate turnover during prolonged exercise in man. J. Clin. Invest. 53, 1080–1090 (1974).
Zimmermann, R. et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004).
Vaughan, M., Berger, J. E. & Steinberg, D. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J. Biol. Chem. 239, 401–409 (1964).
Perdikari, A. et al. Visualization of sympathetic neural innervation in human white adipose tissue. Open Biol. 12, 210345 (2022).
Dodt, C., Lönnroth, P., Fehm, H. L. & Elam, M. Intraneural stimulation elicits an increase in subcutaneous interstitial glycerol levels in humans. J. Physiol. 521, 545–552 (1999).
Barbe, P., Millet, L., Galitzky, J., Lafontan, M. & Berlan, M. In situ assessment of the role of the β1, β2- and β3-adrenoceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br. J. Pharmacol. 117, 907–913 (1996).
Stich, V. et al. Activation of antilipolytic α2-adrenergic receptors by epinephrine during exercise in human adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 277, R1076–R1083 (1999).
Flechtner-Mors, M. et al. Sympathetic regulation of glucose uptake by the α1-adrenoceptor in human obesity. Obes. Res. 12, 612–620 (2004).
Hjemdahl, P., Linde, B., Daleskog, M. & Belfrage, E. Sympatho-adrenal regulation of adipose tissue blood flow in dog and man. Gen. Pharmacol. Vasc. Syst. 14, 175–177 (1983).
Samra, J. S. et al. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 271, E834–E839 (1996).
Giordano, A. et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1243–R1255 (2006).
Kreier, F. et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat — functional implications. J. Clin. Invest. 110, 1243–1250 (2002).
Kreier, F. & Buijs, R. M. Evidence for parasympathetic innervation of white adipose tissue, clearing up some vagaries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R548–R549 (2007).
Giordano, A. et al. Reply to Kreier and Buijs: no sympathy for the claim of parasympathetic innervation of white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R550–R552 (2007).
Jensen, T. E. & Richter, E. A. Regulation of glucose and glycogen metabolism during and after exercise. J. Physiol. 590, 1069–1076 (2012).
Guilherme, A., Henriques, F., Bedard, A. H. & Czech, M. P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 15, 207–225 (2019).
Wang, Y. et al. The role of somatosensory innervation of adipose tissues. Nature 609, 569–574 (2022).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Carpentier, A. C. & Blondin, D. P. Human brown adipose tissue is not enough to combat cardiometabolic diseases. J. Clin. Investig. 133, e175288 (2023).
Cypess, A. M. et al. Emerging debates and resolutions in brown adipose tissue research. Cell Metab. 37, 12–33 (2025).
Cypess, A. M. et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 130, 2209–2219 (2020).
Jensen, M. D. Brown adipose tissue — not as hot as we thought. J. Physiol. 593, 489–490 (2015).
Ferrannini, E. et al. The disposal of an oral glucose load in healthy subjects: a quantitative study. Diabetes 34, 580–588 (1985).
Roatta, S. & Passatore, M. In Encyclopedia of Neuroscience (eds Binder, M. D., Hirokawa, N. & Windhorst, U.) 250–253 (Springer, 2008).
Nonogaki, K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43, 533–549 (2000).
Shimazu, T., Sudo, M., Minokoshi, Y. & Takahashi, A. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res. Bull. 27, 501–504 (1991).
Seoane-Collazo, P. et al. Hypothalamic-autonomic control of energy homeostasis. Endocrine 50, 276–291 (2015).
Ibeas, K., Herrero, L., Mera, P. & Serra, D. Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation. Biochemical Pharmacol. 190, 114640 (2021).
Minokoshi, Y., Haque, M. S. & Shimazu, T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48, 287–291 (1999).
Haque, M. S. et al. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48, 1706–1712 (1999).
Shiuchi, T. et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 10, 466–480 (2009).
Patel, P. N., Horenstein, M. S. & Zwibel, H. Exercise Physiology (StatPearls Publishing, 2025).
Suh, S.-H., Paik, I.-Y. & Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cell 23, 272–279 (2007).
Coker, R. H. & Kjaer, M. Glucoregulation during exercise: the role of the neuroendocrine system. Sports Med. 35, 575–583 (2005).
Kjaer, M., Kiens, B., Hargreaves, M. & Richter, E. A. Influence of active muscle mass on glucose homeostasis during exercise in humans. J. Appl. Physiol. 71, 552–557 (1991).
Wasserman, D. H. Regulation of glucose fluxes during exercise in the postabsorptive state. Annu. Rev. Physiol. 57, 191–218 (1995).
Christensen, N. J. & Galbo, H. Sympathetic nervous activity during exercise. Annu. Rev. Physiol. 45, 139–153 (1983).
Hearon, C. M. & Dinenno, F. A. Regulation of skeletal muscle blood flow during exercise in ageing humans. J. Physiol. 594, 2261–2273 (2016).
Jamerson, K. A., Julius, S., Gudbrandsson, T., Andersson, O. & Brant, D. O. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension 21, 618–623 (1993).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Chen, Z.-T., Weng, Z.-X., Lin, J. D. & Meng, Z.-X. Myokines: metabolic regulation in obesity and type 2 diabetes. Life Metab. 3, loae006 (2024).
Pedersen, B. K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 15, 383–392 (2019).
Samadian, Z., Samadian, L. & Arabzadeh, E. Exercise training enhances myokine release and reduces brain insulin resistance: insights into muscle-CNS metabolic cross-talk. Metab. Brain Dis. 40, 271 (2025).
Shoelson, S. E. Inflammation and insulin resistance. J. Clin. Investig. 116, 1793–1801 (2006).
Lee, Y. S., Wollam, J. & Olefsky, J. M. An integrated view of immunometabolism. Cell 172, 22–40 (2018).
Roden, M. & Shulman, G. I. The integrative biology of type 2 diabetes. Nature 576, 51–60 (2019).
Li, J. H., Hepworth, M. R. & O’Sullivan, T. E. Regulation of systemic metabolism by tissue-resident immune cell circuits. Immunity 56, 1168–1186 (2023).
Dantzer, R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504 (2018).
Cao, Y., Chen, H. & Yang, J. Neuroanatomy of lymphoid organs: lessons learned from whole-tissue imaging studies. Eur. J. Immunol. 53, 2250136 (2023).
Bellinger, D. L. et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 252, 27–56 (2008).
Ulloa, L. Bioelectronic neuro-immunology: neuronal networks for sympathetic-splenic and vagal-adrenal control. Neuron 111, 10–14 (2023).
Pongratz, G. & Straub, R. H. Chronic effects of the sympathetic nervous system in inflammatory models. Neuroimmunomodulation 30, 113–134 (2023).
Alen, N. V. The cholinergic anti-inflammatory pathway in humans: state-of-the-art review and future directions. Neurosci. Biobehav. Rev. 136, 104622 (2022).
Schiller, M., Ben-Shaanan, T. L. & Rolls, A. Neuronal regulation of immunity: why, how and where? Nat. Rev. Immunol. 21, 20–36 (2021).
Sharma, D. & Farrar, J. D. Adrenergic regulation of immune cell function and inflammation. Semin. Immunopathol. 42, 709–717 (2020).
Kolter, J., Kierdorf, K. & Henneke, P. Origin and differentiation of nerve-associated macrophages. J. Immunol. 204, 271–279 (2020).
Ural, B. B. et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci. Immunol. 5, eaax8756 (2020).
Wang, P. L. et al. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 11, 2552 (2020).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Larabee, C. M., Neely, O. C. & Domingos, A. I. Obesity: a neuroimmunometabolic perspective. Nat. Rev. Endocrinol. 16, 30–43 (2020).
Boura-Halfon, S., Pecht, T., Jung, S. & Rudich, A. Obesity and dysregulated central and peripheral macrophage–neuron cross-talk. Eur. J. Immunol. 49, 19–29 (2019).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Msheik, Z., El Massry, M., Rovini, A., Billet, F. & Desmoulière, A. The macrophage: a key player in the pathophysiology of peripheral neuropathies. J. Neuroinflamm. 19, 97 (2022).
Straub, R. H., Cutolo, M., Buttgereit, F. & Pongratz, G. Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases. J. Intern. Med. 267, 543–560 (2010).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Cailotto, C. et al. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 24, 191 (2012).
Song, K. & Kim, B. S. The peripheral neuroimmune system. J. Leukoc. Biol. 116, 1291–1300 (2024).
Soto-Tinoco, E., Santacruz, E., Basualdo-Sigales, M. D. C., Guerrero-Vargas, N. N. & Buijs, R. M. Time-of-day-dependent gating of the liver-spinal axis initiates an anti-inflammatory reflex in the rat. eNeuro 7, ENEURO.0463-20.2020 (2020).
Buijs, R. M., Van Der Vliet, J., Garidou, M.-L., Huitinga, I. & Escobar, C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS ONE 3, e3152 (2008).
Verlinden, T. J. M. et al. Innervation of the human spleen: a complete hilum-embedding approach. Brain Behav. Immun. 77, 92–100 (2019).
Kelly, M. J., Breathnach, C., Tracey, K. J. & Donnelly, S. C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 3, 100696 (2022).
Herhaus, B., Conrad, R. & Petrowski, K. Effect of a slow-paced breathing with heart rate variability biofeedback intervention on pro-inflammatory cytokines in individuals with panic disorder — a randomized controlled trial. J. Affect. Disord. 326, 132–138 (2023).
Thorp, A. A. & Schlaich, M. P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 341583 (2015).
Russo, B., Menduni, M., Borboni, P., Picconi, F. & Frontoni, S. Autonomic nervous system in obesity and insulin-resistance — the complex interplay between leptin and central nervous system. IJMS 22, 5187 (2021).
Hausdorff, W. P., Caron, M. G. & Lefkowitz, R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 4, 2881–2889 (1990).
Pavlov, V. A. & Tracey, K. J. The vagus nerve and the inflammatory reflex — linking immunity and metabolism. Nat. Rev. Endocrinol. 8, 743–754 (2012).
Le Thuc, O. & García-Cáceres, C. Obesity-induced inflammation: connecting the periphery to the brain. Nat. Metab. 6, 1237–1252 (2024).
Straznicky, N. E. et al. Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes 59, 71–79 (2010).
Bönhof, G. J. et al. High-intensity interval training for 12 weeks improves cardiovascular autonomic function but not somatosensory nerve function and structure in overweight men with type 2 diabetes. Diabetologia 65, 1048–1057 (2022).
Herhaus, B. et al. Effect of 4 weeks resonance frequency breathing on glucose metabolism and autonomic tone in healthy adults. Diabetes Metab. J. 49, 1219–1228 (2025).
Picard, M. et al. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: a systematic review and meta-analysis. PLoS ONE 16, e0251863 (2021).
Penzlin, A. I. et al. Effect of short-term heart rate variability biofeedback on long-term abstinence in alcohol dependent patients — a one-year follow-up. BMC Psychiatry 17, 325 (2017).
Lachowska, K., Bellwon, J., Narkiewicz, K., Gruchała, M. & Hering, D. Long-term effects of device-guided slow breathing in stable heart failure patients with reduced ejection fraction. Clin. Res. Cardiol. 108, 48–60 (2019).
Chazova, I., Almazov, V. A. & Shlyakhto, E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: a comparison with metformin. Diabetes Obes. Metab. 8, 456–465 (2006).
Pavlov, V. A. et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23, 41–45 (2009).
Shikora, S. A. et al. Intermittent vagal nerve block for improvements in obesity, cardiovascular risk factors, and glycemic control in patients with type 2 diabetes mellitus: 2-year results of the VBLOC DM2 study. Obes. Surg. 26, 1021–1028 (2016).
McEvoy, J. W. et al. 2024 ESC guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 45, 3912–4018 (2024).
Austelle, C. W., Cox, S. S., Wills, K. E. & Badran, B. W. Vagus nerve stimulation (VNS): recent advances and future directions. Clin. Auton. Res. 34, 529–547 (2024).
Gordan, R., Gwathmey, J. K. & Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 7, 204–214 (2015).
Kim, S. M. et al. Regulation of renin secretion and expression in mice deficient in β1- and β2-adrenergic receptors. Hypertension 50, 103–109 (2007).
Motiejunaite, J., Amar, L. & Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. Endocrinol. 82, 193–197 (2021).
Proctor, G. B. Muscarinic receptors and salivary secretion. J. Appl. Physiol. 100, 1103–1104 (2006).
Langmead, C. J., Watson, J. & Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 117, 232–243 (2008).
Ehlert, F. J. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci. 74, 355–366 (2003).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Health 5, 258–258 (2017).
La Rovere, M. T., Pinna, G. D. & Raczak, G. Baroreflex sensitivity: measurement and clinical implications. Ann. Noninvasive Electrocardiol. 13, 191–207 (2008).
Swenne, C. A. Baroreflex sensitivity: mechanisms and measurement. Neth. Heart J. 21, 58–60 (2013).
White, D. W., Shoemaker, J. K. & Raven, P. B. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Autonomic Neurosci. Basic Clin. 193, 12–21 (2015).
Macefield, V. G. Recording and quantifying sympathetic outflow to muscle and skin in humans: methods, caveats and challenges. Clin. Auton. Res. 31, 59–75 (2021).
Peaston, R. T. & Weinkove, C. Measurement of catecholamines and their metabolites. Ann. Clin. Biochem. 41, 17–38 (2004).
Eisenhofer, G., Pamporaki, C. & Lenders, J. W. M. Biochemical assessment of pheochromocytoma and paraganglioma. Endocr. Rev. 44, 862–909 (2023).
Acknowledgements
The authors acknowledge the support of an ERC Consolidator grant (CrossPeriBrain, Project 101125605 to M.H.).
Author information
Authors and Affiliations
Contributions
All authors reviewed data for the article. All authors contributed substantially to discussion of the content. S.W., M.N.J., M.E., R.S. and M.H. wrote the article. All authors reviewed and/or edited the manuscript and approved the final version before submission.
Corresponding author
Ethics declarations
Competing interests
M.N.J. reports scientific consultation work for LaVita GmbH. R.W. reports receiving lecture fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi-Aventis, and has served on the advisory board for Akcea Therapeutics, Daiichi Sankyo, Eli Lilly, Novo Nordisk and Sanofi-Aventis. M.H. reports receiving lecture fees from Amryt/Chiesi, AstraZeneca, Bayer, Boehringer Ingelheim, Daichii Sankyo, Lilly, Novartis, Novo Nordisk and Sanofi-Aventis. He has also served on advisory boards for Amryt/Chiesi and Boehringer Ingelheim. He is currently a board member of the German Diabetes Association. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks João M.N. Duarte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
The authors are partly funded by the European Union. The views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wangler, S., Jarczok, M.N., Ennis, M. et al. The autonomic nervous system in the regulation of glucose and lipid metabolism. Nat Rev Endocrinol (2026). https://doi.org/10.1038/s41574-025-01221-w
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41574-025-01221-w