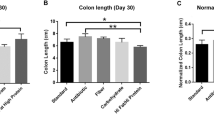
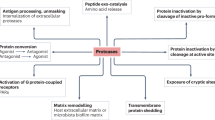
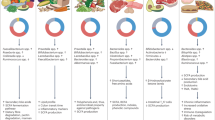
Abstract
Protein is an essential nutrient in the human diet. Global Westernization and modern dietary trends have seen protein become a more substantial contributor to the Western diet, with dietary sources expanding beyond traditional wholefoods to a myriad of processed protein-enriched food products. Although dietary protein is critical for human health, it has also been implicated in colonic health and disease both directly via the microbial fermentation of protein entering the colonic environment and indirectly by affecting the intake of other nutrients in the diet such as fibre. Although protein digestion in the small intestine is highly efficient, there are numerous factors that can influence the capacity for protein digestion and absorption, particularly dietary factors representative of modern-day protein intakes such as high protein diets and food manufacturing. The subsequent fermentation of protein and production of microbial metabolites in the colon is in turn affected by the source of protein entering the colon and the presence of fibre. In this Review, we examine factors that influence human digestion and absorption of protein in the small intestine and protein fermentation in the colon, describing implications for colonic health and disease.
Key points
-
Global Westernization has changed the way protein is consumed in modern-day society with trends focused on high protein diets and processed protein sources.
-
Dietary and non-dietary factors including protein source, fibre intake and medications influence capacity for digestion and absorption of protein in the small intestine and subsequent availability for colonic fermentation.
-
Metabolites produced during colonic protein fermentation have the potential to exert beneficial and/or detrimental effects on the colonic mucosa.
-
Optimization of protein intake requires careful consideration of the effect of protein on colonic health directly via its colonic fermentation and indirectly by its effect on dietary intake of other nutrients.
-
Further research is required to personalize protein recommendations based on genetic, environmental and microbial data to optimize health and minimize the risk of colonic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wu, G. Dietary protein intake and human health. Food Funct. 7, 1251–1265 (2016).
Schmidhuber, J. & Shetty, P. The nutrition transition to 2030. Why developing countries are likely to bear the major burden. Food Econ. 2, 150–166 (2005).
Grigg, D. The pattern of world protein consumption. Geoforum 26, 1–17 (1995).
Campbell, B. et al. International Society of Sports Nutrition position stand: protein and exercise. J. Int. Soc. Sports Nutr. 4, 8 (2007).
World Health Organization. Red and processed meat in the context of health and the environment: many shades of red and green: information brief https://www.who.int/publications/i/item/9789240074828 (WHO, 2023).
Silvester, K. R. & Cummings, J. H. Does digestibility of meat protein help explain large bowel cancer risk? Nutr. Cancer 24, 279–288 (1995).
Magee, E. A., Richardson, C. J., Hughes, R. & Cummings, J. H. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 72, 1488–1494 (2000).
Zannini, E., Sahin, A. W. & Arendt, E. K. Resistant protein: forms and functions. Foods 11, 2759 (2022).
Kato, N. & Iwami, K. Resistant protein; its existence and function beneficial to health. J. Nutr. Sci. Vitaminol. 48, 1–5 (2002).
Windey, K., De Preter, V. & Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 56, 184–196 (2012).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Yao, C. K., Muir, J. G. & Gibson, P. R. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 43, 181–196 (2016).
Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition. WHO Technical Report Series 935 (WHO, 2007).
Day, L., Cakebread, J. A. & Loveday, S. M. Food proteins from animals and plants: differences in the nutritional and functional properties. Trends Food Sci. Technol. 119, 428–442 (2022).
Erickson, R. H. & Kim, Y. S. Digestion and absorption of dietary protein. Annu. Rev. Med. 41, 133–139 (1990).
Goodman, B. E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 34, 44–53 (2010).
Hoffer, L. J. Human protein and amino acid requirements. JPEN J. Parenter. Enter. Nutr. 40, 460–474 (2016).
Australian Bureau of Statistics. Australian Health Survey: Nutrition First Results-Foods and Nutrients https://www.abs.gov.au/statistics/health/food-and-nutrition/food-and-nutrients/2011-12 (Australian Bureau of Statistics, 2014).
Australian Bureau of Statistics. Australian Health Survey: Food and Nutrients https://www.abs.gov.au/statistics/health/food-and-nutrition/food-and-nutrients/latest-release (Australian Bureau of Statistics, 2025).
International Food Information Council. Americans’ Perceptions of Protein https://ific.org/wp-content/uploads/2025/07/IFIC-Spotlight-Survey-Protein-Perceptions.pdf (2025).
Wilson, B. Protein Mania: the Rich World’s New Diet Obsession https://www.theguardian.com/news/2019/jan/04/protein-mania-the-rich-worlds-new-diet-obsession (The Guardian, 2019).
Arenas-Jal, M., Suñé-Negre, J., Pérez-Lozano, P. & García-Montoya, E. Trends in the food and sports nutrition industry: a review. Crit. Rev. Food Sci. Nutr. 60, 2405–2421 (2020).
Chapple, C. I., Burnett, A. J., Woods, J. L. & Russell, C. G. A cross-sectional study of sports food consumption patterns, experiences, and perceptions amongst non-athletes in Australia. Nutrients 16, 1101 (2024).
Cordain, L. et al. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 81, 341–354 (2005).
Alae-Carew, C. et al. The role of plant-based alternative foods in sustainable and healthy food systems: Consumption trends in the UK. Sci. Total. Env. 807, 151041, (2022).
O’Keefe, S. J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 13, 691–706 (2016).
World Health Organization. Colorectal Cancer https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (WHO, 2023).
Singh, R. K. et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15, 73 (2017).
Dong, C. et al. Meat intake is associated with a higher risk of ulcerative colitis in a large European prospective cohort study. J. Crohns Colitis 16, 1187–1196 (2022).
Larsson, S. C. & Wolk, A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int. J. Cancer 119, 2657–2664 (2006).
Blachier, F. et al. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol 320, 125–135 (2021).
Gill, S. K., Rossi, M., Bajka, B. & Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 18, 101–116 (2021).
Petroski, W. & Minich, D. M. Is there such a thing as “anti-nutrients”? A narrative review of perceived problematic plant compounds. Nutrients 12, 2929 (2020).
Gibson, J. A., Sladen, G. E. & Dawson, A. M. Protein absorption and ammonia production: the effects of dietary protein and removal of the colon. Br. J. Nutr. 35, 61–65 (1976).
Moreno-Pérez, D. et al. Effect of a protein supplement on the gut microbiota of endurance athletes: a randomized, controlled, double-blind pilot study. Nutrients 10, 337 (2018).
Dale, H. F. et al. Effects of a cod protein hydrolysate supplement on symptoms, gut integrity markers and fecal fermentation in patients with irritable bowel syndrome. Nutrients 11, 1635 (2019).
Geypens, B. et al. Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 41, 70–76 (1997).
Silvester, K. R., Bingham, S. A., Pollock, J. R., Cummings, J. H. & O’Neill, I. K. Effect of meat and resistant starch on fecal excretion of apparent N-nitroso compounds and ammonia from the human large bowel. Nutr. Cancer 29, 13–23 (1997).
Bingham, S. A. et al. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis 17, 515–523 (1996).
Cummings, J. H., Hill, M. J., Bone, E. S., Branch, W. J. & Jenkins, D. J. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 32, 2094–2101 (1979).
Mitchell, S. et al. A period of 10 weeks of increased protein consumption does not alter faecal microbiota or volatile metabolites in healthy older men: a randomised controlled trial. J. Nutr. Sci. 9, e25 (2020).
Korpela, K. Diet, microbiota, and metabolic health: trade-off between saccharolytic and proteolytic fermentation. Annu. Rev. Food Sci. Technol. 9, 65–84 (2018).
Rasheed, F., Markgren, J., Hedenqvist, M. & Johansson, E. Modeling to understand plant protein structure-function relationships — implications for seed storage proteins. Molecules 25, 873 (2020).
Sá, A. G. A., Moreno, Y. M. F. & Carciofi, B. A. M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 60, 3367–3386 (2020).
Guillin, F. M. et al. Real ileal amino acid digestibility of pea protein compared to casein in healthy humans: a randomized trial. Am. J. Clin. Nutr. 115, 353–363 (2022).
Bos, C. et al. Assessment of net postprandial protein utilization of 15N-labelled milk nitrogen in human subjects. Br. J. Nutr. 81, 221–226 (1999).
Tessier, R. et al. Digestive and metabolic bioavailability in healthy humans of 15N-labeled rapeseed and flaxseed protein incorporated in biscuits. Am. J. Clin. Nutr. 117, 896–902 (2023).
Kashyap, S. et al. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am. J. Clin. Nutr. 110, 873–882 (2019).
Evenepoel, P. et al. Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J. Nutr. 128, 1716–1722 (1998).
Teigen, L. et al. Differential hydrogen sulfide production by a human cohort in response to animal- and plant-based diet interventions. Clin. Nutr. 41, 1153–1162 (2022).
Beaumont, M. et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 106, 1005–1019 (2017).
Patel, K. P., Luo, F. J. G., Plummer, N. S., Hostetter, T. H. & Meyer, T. W. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin. J. Am. Soc. Nephrol. 7, 982–988 (2012).
Eggum, B. O. in Dietary Fibre — A Component of Food: Nutritional Function in Health and Disease (eds Schweizer, T. F. & Edwards, C. A.) 153–165 (Springer, 1992).
Abrahamsson, M., Aman, P., Hallmans, G., Zhang, J. X. & Lundin, E. Excretion of amino acid residues from diets based on low-fibre wheat or high-fibre rye bread in human subjects with ileostomies. Eur. J. Clin. Nutr. 49, 589–595 (1995).
Macfarlane, G., Gibson, G. & Cummings, J. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72, 57–64 (1992).
Cummings, J. H. & Branch, W. J. in Dietary Fiber: Basic and Clinical Aspects (eds Vahouny, G. V. & Kritchevsky, D.) 131–149 (Springer, 1986).
Boets, E. et al. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients 7, 8916–8929 (2015).
Yao, C. K. et al. Modulation of colonic hydrogen sulfide production by diet and mesalazine utilizing a novel gas-profiling technology. Gut Microbes 9, 510–522 (2018).
Muir, J. G. et al. Combining wheat bran with resistant starch has more beneficial effects on fecal indexes than does wheat bran alone. Am. J. Clin. Nutr. 79, 1020–1028 (2004).
Lewis, S., Brazier, J., Beard, D., Nazem, N. & Proctor, D. Effects of metronidazole and oligofructose on faecal concentrations of sulphate-reducing bacteria and their activity in human volunteers. Scand. J. Gastroenterol. 40, 1296–1303 (2005).
Birkett, A., Muir, J., Phillips, J., Jones, G. & O’Dea, K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 63, 766–772 (1996).
McIntyre, A., Young, G. P., Taranto, T., Gibson, P. R. & Ward, P. B. Different fibers have different regional effects on luminal contents of rat colon. Gastroenterology 101, 1274–1281 (1991).
Govers, M., Gannon, N., Dunshea, F., Gibson, P. & Muir, J. Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: a study in pigs. Gut 45, 840–847 (1999).
So, D. et al. Detection of changes in regional colonic fermentation in response to supplementing a low FODMAP diet with dietary fibres by hydrogen concentrations, but not by luminal pH. Aliment. Pharmacol. Ther. 58, 417–428 (2023).
Meade, S. J., Reid, E. A. & Gerrard, J. A. The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 88, 904–922 (2005).
Loveday, S. M. Protein digestion and absorption: the influence of food processing. Nutr. Res. Rev. 36, 544–559 (2022).
Prodhan, U. K. et al. Comparable postprandial amino acid and gastrointestinal hormone responses to beef steak cooked using different methods: a randomised crossover trial. Nutrients 12, 380 (2020).
Oberli, M. et al. High true ileal digestibility but not postprandial utilization of nitrogen from bovine meat protein in humans is moderately decreased by high-temperature, long-duration cooking. J. Nutr. 145, 2221–2228 (2015).
Liu, K., Zheng, J. & Chen, F. Heat-induced changes in the physicochemical properties and in vitro digestibility of rice protein fractions. J. Food Sci. Technol. 58, 1368–1377 (2021).
Accardo, F., Leni, G., Tedeschi, T., Prandi, B. & Sforza, S. Structural and chemical changes induced by temperature and pH hinder the digestibility of whey proteins. Food Chem. 387, 132884 (2022).
Trommelen, J. et al. Casein protein processing strongly modulates post-prandial plasma amino acid responses in vivo in humans. Nutrients 12, 2299 (2020).
Loveday, S. M., Peram, M. R., Singh, H., Ye, A. & Jameson, G. B. Digestive diversity and kinetic intrigue among heated and unheated β-lactoglobulin species. Food Funct. 5, 2783–2791 (2014).
Drummond, E., O’Sullivan, V., Sc Sri Harsha, P., Brennan, L. & Horner, K. Effects of a casein hydrolysate versus intact casein on gastric emptying and amino acid responses. Eur. J. Nutr. 58, 955–964 (2019).
Calbet, J. A. & Holst, J. J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 43, 127–139 (2004).
Koopman, R. et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 90, 106–115 (2009).
Aljahdali, N. & Carbonero, F. Impact of Maillard reaction products on nutrition and health: current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 59, 474–487 (2019).
Murray, M. et al. Reduced growth, altered gut microbiome and metabolite profile, and increased chronic kidney disease risk in young pigs consuming a diet containing highly resistant protein. Front. Nutr. 9, 816749 (2022).
Seiquer, I. et al. Diets rich in Maillard reaction products affect protein digestibility in adolescent males aged 11–14 y. Am. J. Clin. Nutr. 83, 1082–1088 (2006).
Hellwig, M. et al. Stability of individual Maillard reaction products in the presence of the human colonic microbiota. J. Agric. Food Chem. 63, 6723–6730 (2015).
Mills, D. et al. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. J. Appl. Microbiol. 105, 706–714 (2008).
Yuan, X. et al. Accumulation and effects of dietary advanced glycation end products on the gastrointestinal tract in rats. Int. J. Food Sci. Technol. 53, 2273–2281 (2018).
Mastrocola, R. et al. Effects of exogenous dietary advanced glycation end products on the cross-talk mechanisms linking microbiota to metabolic inflammation. Nutrients 12, 2497 (2020).
Shanika, L. G. T., Reynolds, A., Pattison, S. & Braund, R. Proton pump inhibitor use: systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 79, 1159–1172 (2023).
Evenepoel, P. Alteration in digestion and absorption of nutrients during profound acid suppression. Best. Pract. Res. Clin. Gastroenterol. 15, 539–551 (2001).
Evenepoel, P. et al. Evidence for impaired assimilation and increased colonic fermentation of protein, related to gastric acid suppression therapy. Aliment. Pharmacol. Ther. 12, 1011–1019 (1998).
Kostiuchenko, O. et al. Effects of proteases from pineapple and papaya on protein digestive capacity and gut microbiota in healthy C57BL/6 mice and dose-manner response on mucosal permeability in human reconstructed intestinal 3D tissue model. Metabolites 12, 1027 (2022).
Wiboonsirikul, J., Klahan, R. & Khuwijitjaru, P. Extraction of crude bromelain from pineapple (Ananas comosus L.) fruit waste and it’s in vitro protein digestibility. J. Agric. Sci. 19, 73–88 (2024).
Lee, S. Y. et al. Methods for improving meat protein digestibility in older adults. J. Anim. Sci. Technol. 65, 32–56 (2023).
Garces Ortega, M. Effect of Proteolytic Enzyme and Fiber of Papaya Fruit on Human Digestive Health Thesis, Univ. Illinois at Urbana-Champaign (2012).
Park, S. et al. The impact of Hayward green kiwifruit on dietary protein digestion and protein metabolism. Eur. J. Nutr. 60, 1141–1148 (2021).
Jäger, R. et al. Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob. Proteins 12, 1330–1339 (2020).
Walden, K. E. et al. Probiotic BC30 improves amino acid absorption from plant protein concentrate in older women. Probiotics Antimicrob. Proteins 16, 125–137 (2022).
Stecker, R. A. et al. Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein. Nutr. Metab. 17, 93 (2020).
Gilbert, M. S., Ijssennagger, N., Kies, A. K. & van Mil, S. W. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G159–G170 (2018).
Smirnov, K. S. et al. Challenges of metabolomics in human gut microbiota research. Int. J. Med. Microbiol. 306, 266–279 (2016).
Rhodes, J. M. Nutrition and gut health: the impact of specific dietary components — it’s not just five-a-day. Proc. Nutr. Soc. 80, 9–18 (2021).
Gibson, P. R., Halmos, E. P. & Muir, J. G. FODMAPS, prebiotics and gut health — the FODMAP hypothesis revisited. Aliment. Pharmacol. Ther. 52, 233–246 (2020).
Morita, A., Tsao, D. & Kim, Y. S. Effect of sodium butyrate on alkaline phosphatase in HRT-18, a human rectal cancer cell line. Cancer Res. 42, 4540–4545 (1982).
Macfarlane, G. & Allison, C. Utilisation of protein by human gut bacteria. FEMS Microbiol. Ecol. 2, 19–24 (1986).
Vancamelbeke, M. & Vermeire, S. The intestinal barrier: a fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 11, 821–834 (2017).
Buret, A. G., Allain, T., Motta, J.-P. & Wallace, J. L. Effects of hydrogen sulfide on the microbiome: from toxicity to therapy. Antioxid. Redox Signal. 36, 211–219 (2022).
Mutuyemungu, E., Singh, M., Liu, S. & Rose, D. J. Intestinal gas production by the gut microbiota: a review. J. Funct. Foods 100, 105367 (2023).
Suarez, F., Springfield, J. & Levitt, M. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut 43, 100–104 (1998).
Tangerman, A. & Winkel, E. G. Volatile sulfur compounds as the cause of bad breath: a review. Phosphorus Sulfur. Silicon Relat. Elem. 188, 396–402 (2013).
Trabue, S., Kerr, B., Scoggin, K., Andersen, D. & Van Weelden, M. Swine diets impact manure characteristics and gas emissions: part I protein level. Sci. Total. Env. 755, 142528 (2021).
Peters, V. et al. Western and carnivorous dietary patterns are associated with greater likelihood of IBD development in a large prospective population-based cohort. J. Crohns Colitis 16, 931–939 (2022).
Jowett, S. et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 53, 1479–1484 (2004).
Zinger, A., Barnes, E. L., Weisbein, L., Kappelman, M. & Micic, D. High red meat consumption is associated with greater risk of ulcerative colitis flare [abstract S61]. Am. J. Gastroenterol. 118, S17 (2023).
Pitcher, M. & Cummings, J. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut 39, 1–4 (1996).
Roediger, W. E. Decreased sulphur aminoacid intake in ulcerative colitis. Lancet 351, 1555 (1998).
Zhu, S. et al. Composition and diverse differences of intestinal microbiota in ulcerative colitis patients. Front. Cell Infect. Microbiol. 12, 953962 (2022).
Rowan, F. et al. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon. Rectum 53, 1530–1536 (2010).
James, S. L. et al. Abnormal fibre usage in UC in remission. Gut 64, 562–570 (2015).
Armstrong, H. K. et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 164, 228–240 (2023).
Khalil, N. A., Walton, G. E., Gibson, G. R., Tuohy, K. M. & Andrews, S. C. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int. J. Food Sci. Nutr. 65, 79–88 (2014).
Gibson, G., Cummings, J. & Macfarlane, G. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol. Lett. 86, 103–111 (1991).
Levine, J., Ellis, C. J., Furne, J. K., Springfield, J. & Levitt, M. D. Fecal hydrogen sulfide production in ulcerative colitis. Am. J. Gastroenterol. 93, 83–87 (1998).
De Preter, V. et al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm. Bowel Dis. 18, 1127–1136 (2012).
Roediger, W. nitric oxide from dysbiotic bacterial respiration of nitrate in the pathogenesis and as a target for therapy of ulcerative colitis. Aliment. Pharmacol. Ther. 27, 531–541 (2008).
Gibson, P., Van de Pol, E., Barratt, P. & Doe, W. Ulcerative colitis — a disease characterised by the abnormal colonic epithelial cell? Gut 29, 516–521 (1988).
Chiba, M. et al. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: a single-group trial. Perm. J. 23, 18–220 (2019).
Day, A. S. et al. Therapeutic potential of the 4 strategies to SUlfide-REduction (4-SURE) diet in adults with mild to moderately active ulcerative colitis: an open-label feasibility study. J. Nutr. 152, 1690–1701 (2022).
Sarbagili-Shabat, C. et al. A novel UC exclusion diet and antibiotics for treatment of mild to moderate pediatric ulcerative colitis: a prospective open-label pilot study. Nutrients 13, 3736 (2021).
Louis, P., Hold, G. L. & Flint, H. J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672 (2014).
Schmitt, M. & Greten, F. R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 21, 653–667 (2021).
Joosen, A. M. et al. Effect of dietary meat and fish on endogenous nitrosation, inflammation and genotoxicity of faecal water. Mutagenesis 25, 243–247 (2010).
Aune, D. et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343, d6617 (2011).
So, D., Gibson, P. R., Muir, J. G. & Yao, C. K. Dietary fibres and IBS: translating functional characteristics to clinical value in the era of personalised medicine. Gut 70, 2383–2394 (2021).
Zeng, H., Lazarova, D. L. & Bordonaro, M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol. 6, 41–51 (2014).
Li, Y. & Tong, W. D. Association between dietary protein intake and constipation: data from the National Health and Nutrition Examination Survey 2005–2010. Neurogastroenterol. Motil. 36, e14795 (2024).
Austin, G. L. et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 7, 706–708.e1 (2009).
Halmos, E. P., Power, V. A., Shepherd, S. J., Gibson, P. R. & Muir, J. G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146, 67–75 (2014).
Ramani, A., Hazra, T., Mudgil, S. & Mudgil, D. Emerging potential of whey proteins in prevention of cancer. Food Humanity 2, 100199 (2024).
Brown, T. D., Whitehead, K. A. & Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 5, 127–148 (2020).
Yao, C. K. et al. Effects of fiber intake on intestinal pH, transit, and predicted oral mesalamine delivery in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 36, 1580–1589 (2021).
Bischoff, S. C. et al. ESPEN practical guideline: clinical nutrition in inflammatory bowel disease. Clin. Nutr. 39, 632–653 (2020).
Singar, S., Nagpal, R., Arjmandi, B. H. & Akhavan, N. S. Personalized nutrition: tailoring dietary recommendations through genetic insights. Nutrients 16, 2673 (2024).
Allaband, C. et al. Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin. Gastroenterol. Hepatol. 17, 218–230 (2019).
Lagoumintzis, G. & Patrinos, G. P. Triangulating nutrigenomics, metabolomics and microbiomics toward personalized nutrition and healthy living. Hum. Genomics 17, 109 (2023).
Jaskiewicz, J. et al. Catabolism of isobutyrate by colonocytes. Arch. Biochem. Biophys. 327, 265–270 (1996).
Li, X., Zhang, B., Hu, Y. & Zhao, Y. New insights into gut-bacteria-derived indole and its derivatives in intestinal and liver diseases. Front. Pharmacol. 12, 769501 (2021).
Zhang, Y. et al. Unraveling p-cresol: from biosynthesis to biological and biochemical activities. Front. Pharmacol. 16, 1665421 (2025).
Davila, A.-M. et al. Re-print of “Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host”. Pharmacol. Res. 69, 114–126 (2013).
Ichikawa, H. & Sakata, T. Stimulation of epithelial cell proliferation of isolated distal colon of rats by continuous colonic infusion of ammonia or short-chain fatty acids is nonadditive. J. Nutr. 128, 843–847 (1998).
Bradley III, E., Isaacs, J., Hersh, T., Davidson, E. D. & Millikan, W. Nutritional consequences of total gastrectomy. Ann. Surg. 182, 415–429 (1975).
Crenn et al. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut 53, 1279–1286 (2004).
Tappenden, K. A. Intestinal adaptation following resection. JPEN J. Parenter. Enter. Nutr. 38, 23S–31S (2014).
Keller, J., Panter, H. & Layer, P. Management of the short bowel syndrome after extensive small bowel resection. Best. Pract. Res. Clin. Gastroenterol. 18, 977–992 (2004).
Luscombe-Marsh, N. D. et al. Plasma free amino acid responses to intraduodenal whey protein, and relationships with insulin, glucagon-like peptide-1 and energy intake in lean healthy men. Nutrients 8, 1, 4 (2016).
Diether, N. E. & Willing, B. P. Microbial fermentation of dietary protein: an important factor in diet–microbe–host interaction. Microorganisms 7, 19 (2019).
Dhakal, S., Moazzami, Z., Perry, C. & Dey, M. Effects of lean pork on microbiota and microbial-metabolite trimethylamine-N-oxide: a randomized controlled non-inferiority feeding trial based on the dietary guidelines for Americans. Mol. Nutr. Food Res. 66, e2101136 (2022).
Abdallah, A., Elemba, E., Zhong, Q. & Sun, Z. Gastrointestinal gut microbiota: with special emphasis on host nutrition. Curr. Protein Pept. Sci. 21, 785–798 (2020).
Freeman, H. J. & Kim, Y. S. Digestion and absorption of protein. Annu. Rev. Med. 29, 99–116 (1978).
Trommelen, J., Tomé, D. & van Loon, L. J. Gut amino acid absorption in humans: concepts and relevance for postprandial metabolism. Clin. Nutr. Open. Sci. 36, 43–55 (2021).
Ambühl, P. M. Protein intake in renal and hepatic disease. Int. J. Vitam. Nutr. Res. 81, 162–172 (2011).
Wu, G. Amino acids: metabolism, functions, and nutrition. Amino acids 37, 1–17 (2009).
Gaffney-Stomberg, E., Insogna, K. L., Rodriguez, N. R. & Kerstetter, J. E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 57, 1073–1079 (2009).
Muscaritoli, M. et al. ESPEN practical guideline: clinical nutrition in cancer. Clin. Nutr. 40, 2898–2913 (2021).
Hodgkinson, S. M. & Darragh, A. J. Quantifying the digestibility of dietary protein. J. Nutr. 130, 1850S–1856S (2000).
Bandyopadhyay, S. et al. Evaluation of protein quality in humans and insights on stable isotope approaches to measure digestibility — a review. Adv. Nutr. 13, 1131–1143 (2022).
Adhikari, S., Schop, M., de Boer, I. J. M. & Huppertz, T. Protein quality in perspective: a review of protein quality metrics and their applications. Nutrients 14, 947 (2022).
Thwaites, P. A. et al. Current status and future directions of ingestible electronic devices in gastroenterology. Aliment. Pharmacol. Ther. 59, 459–474 (2024).
Acknowledgements
The authors acknowledge grant funding and academic support from The Hospital Research Foundation Group and Michelle McGrath Fellowship. The authors acknowledge A. Holasek from the Central Adelaide Local Health Network Library service who provided support with the practicalities of literature searching. The figures were originally developed with BioRender before being redrawn; Supplementary Fig 1 was created with BioRender; Mathias, R. (2025) https://BioRender.com/v5telb9.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. R.H.D. wrote the original article and created tables and figures. All authors made substantial contributions to discussion of content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.V.B. has received grant, research support or speaker fees (all paid to his employer for research support) from AbbVie, Ferring, Janssen, Shire, Takeda, GSK and Emerge Health; and is a shareholder in Biomebank. P.R.G. is a consultant or advisory board member for Anatara, Atmo Biosciences, Topas and Comvita; has received research grants for investigator-driven studies from Mindset Health, and speaker honoraria from Dr Falk Pharma and Mindset Health Pty.; and is a shareholder in Atmo Biosciences. His salary is derived from sales of a digital application (Monash University FODMAP diet app), patient booklets cookbooks and online courses, all of which relate to the low FODMAP diet therapy. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Alan Mackie, Kaitlin Day and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
The electronic databases Medline, Embase, Emcare and Cochrane Library were searched up to 18 November 2025 to retrieve articles. A comprehensive search strategy using both keywords and MeSH terms was developed to identify a broad scope of potentially relevant articles (Supplementary Table 5). Search terms and synonyms to signify ’Protein‘, ’Digestion‘, ’Absorption‘ and ’Fermentation‘ were used. No limits were applied to the search strategy. However, a range of synonyms for ’animals‘ and ’paediatrics‘ were used in attempt to refine the search to human adults. Citations from each database were exported into EndNote 20 and then Covidence where duplicates were removed. The resulting 8,331 articles were screened by title and abstract to locate primary studies and reviews detailing digestion, absorption or fermentation of protein in the human gastrointestinal tract. Studies in animals, in vitro or simulated digestion, and studies in infants and children were excluded. Additional papers including animal or in vitro data relevant to the concepts discussed in this paper were subsequently included. Full-text articles were screened to determine relevance for inclusion in this narrative review.
Supplementary information
Rights and permissions
About this article
Cite this article
Davis, R.H., Bryant, R.V., Gibson, P.R. et al. The fate of dietary protein in the gastrointestinal tract and implications for colonic disease. Nat Rev Gastroenterol Hepatol (2026). https://doi.org/10.1038/s41575-026-01173-0
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41575-026-01173-0