Abstract
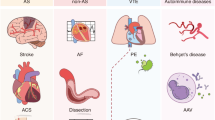
Periodontitis, a major inflammatory disease of the oral mucosa, is epidemiologically associated with other chronic inflammation-driven disorders, including cardio-metabolic, neurodegenerative and autoimmune diseases and cancer. Emerging evidence from interventional studies indicates that local treatment of periodontitis ameliorates surrogate markers of comorbid conditions. The potential causal link between periodontitis and its comorbidities is further strengthened by recent experimental animal studies establishing biologically plausible and clinically consistent mechanisms whereby periodontitis could initiate or aggravate a comorbid condition. This multi-faceted ‘mechanistic causality’ aspect of the link between periodontitis and comorbidities is the focus of this Review. Understanding how certain extra-oral pathologies are affected by disseminated periodontal pathogens and periodontitis-associated systemic inflammation, including adaptation of bone marrow haematopoietic progenitors, may provide new therapeutic options to reduce the risk of periodontitis-associated comorbidities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Genco, R. J. & Sanz, M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol. 2000 83, 7–13 (2020).
Potempa, J., Mydel, P. & Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 606–620 (2017).
Hajishengallis, G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44 (2015).
Acharya, C., Sahingur, S. E. & Bajaj, J. S. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight 2, e94416 (2017).
Schenkein, H. A., Papapanou, P. N., Genco, R. & Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. 2000 83, 90–106 (2020).
Kitamoto, S. et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182, 447–462 (2020). This study elegantly shows that oral bacterial-specific TH17 cells, which expand during experimental periodontitis, are instructed to migrate to the gut, where they are activated by translocated (via the gastrointestinal route) oral bacteria and contribute to the development of colitis.
Abed, J. et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 20, 215–225 (2016). This paper explains F. nucleatum’s tropism to CRC. The bacterium expresses a Gal-GalNAc-binding lectin (Fap2) that can bind to the host polysaccharide Gal-GalNAc, which is highly expressed in human CRC.
Atarashi, K. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 (2017). This is the first paper to show that ectopic gut colonization by bacteria of oral origin results in expansion of colitogenic T cells and promotion of colitis in a susceptible host.
Blasco-Baque, V. et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 66, 872–885 (2017).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015). This study identifies an important immune evasion mechanism by F. nucleatum, which uses its Fap2 adhesin to activate the inhibitory immunoreceptor TIGIT, which disables the tumour-killing activity of T cells and natural killer cells.
Dominy, S. S. et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5, eaau3333 (2019). This study provides strong clinical and experimental evidence that the oral pathogen P. gingivalis can infect the brain and contribute to inflammatory pathology in Alzheimer disease.
Konig, M. F. et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 8, 369ra176 (2016). In this study, the periodontal pathogen A. actinomycetemcomitans is shown to induce the generation of citrullinated autoantigens involved in rheumatoid arthritis through the action of its pore-forming leukotoxin A (LtxA) on neutrophils. Anti-LtxA antibodies are strongly associated with ACPA positivity in patients with rheumatoid arthritis.
Meghil, M. M. et al. Disruption of immune homeostasis in human dendritic cells via regulation of autophagy and apoptosis by Porphyromonas gingivalis. Front. Immunol. 10, 2286 (2019).
Sato, K. et al. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep. 7, 6955 (2017).
Farrugia, C. et al. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. https://doi.org/10.1111/febs.15486 (2020).
Zhao, Y. et al. Characterization and regulation of osteoclast precursors following chronic Porphyromonas gingivalis infection. J. Leukoc. Biol. 108, 1037–1050 (2020). This investigation identifies a mechanism whereby the bone marrow may link periodontitis to other bone loss disorders. Specifically, periodontal bacteria-induced serum IL-6 acts in the bone marrow to promote the expansion and osteoclastogenic lineage bias of a precursor population, which can traffic to sites of bone resorption and differentiate into mature osteoclasts.
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Chavakis, T., Mitroulis, I. & Hajishengallis, G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 20, 802–811 (2019).
D’Aiuto, F. et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 6, 954–965 (2018). A well-designed and executed randomized controlled trial showing favourable effects of local periodontal treatment on systemic inflammatory markers, glycaemic control and vascular and kidney functions in patients with type 2 diabetes mellitus.
Ishai, A. et al. Periodontal disease associates with arterial inflammation via potentiation of a hematopoietic–arterial axis. JACC Cardiovasc. Imaging 12, 2271–2273 (2019). An important clinical–imaging study that correlates periodontal metabolic activity/inflammation with haematopoietic tissue activity and arterial inflammation, thus supporting the hypothesis derived from experimental studies that the inflammatory adaptation of haematopoietic progenitor cells in the bone marrow may link different chronic inflammatory diseases.
Teles, R. & Wang, C.-Y. Mechanisms involved in the association between peridontal diseases and cardiovascular disease. Oral Dis. 17, 450–461 (2011).
Bajaj, J. S. et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G824–G837 (2018).
Xiao, E. et al. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 22, 120–128 e124 (2017).
Teles, F., Wang, Y., Hajishengallis, G., Hasturk, H. & Marchesan, J. Impact of systemic factors in shaping the periodontal microbiome. Periodontol. 2000 85, 126–160 (2021).
Monsarrat, P. et al. Clinical research activity in periodontal medicine: a systematic mapping of trial registers. J. Clin. Periodontol. 43, 390–400 (2016).
Rydén, L. et al. Periodontitis increases the risk of a first myocardial infarction. Circulation 133, 576–583 (2016).
Rodríguez-Lozano, B. et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case–control study. Arthritis Res. Ther. 21, 27 (2019).
Genco, R. J. & Van Dyke, T. E. Prevention: reducing the risk of CVD in patients with periodontitis. Nat. Rev. Cardiol. 7, 479–480 (2010).
D’Aiuto, F., Orlandi, M. & Gunsolley, J. C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Clin. Periodontol. 40, S85–S105 (2013).
H. Bokhari, S. A. et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J. Clin. Periodontol. 39, 1065–1074 (2012).
de Oliveira, C., Watt, R. & Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish health survey. Br. Med. J. 340, c2451 (2010).
Tonetti, M. S. Periodontitis and risk for atherosclerosis: an update on intervention trials. J. Clin. Periodontol. 36, 15–19 (2009).
Türer Ç, C., Durmuş, D., Balli, U. & Güven, B. Effect of non-surgical periodontal treatment on gingival crevicular fluid and serum endocan, vascular endothelial growth factor-A, and tumor necrosis factor-alpha levels. J. Periodontol. 88, 493–501 (2017).
Mammen, M. J., Scannapieco, F. A. & Sethi, S. Oral–lung microbiome interactions in lung diseases. Periodontol. 2000 83, 234–241 (2020).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Arimatsu, K. et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 4, 4828 (2014).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Norata, G. D. et al. The cellular and molecular basis of translational immunometabolism. Immunity 43, 421–434 (2015).
Orlandi, M., Graziani, F. & D’Aiuto, F. Periodontal therapy and cardiovascular risk. Periodontol. 2000 83, 107–124 (2020).
Genco, R. J., Graziani, F. & Hasturk, H. Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol. 2000 83, 59–65 (2020).
Jepsen, S., Suvan, J. & Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol. 2000 83, 125–153 (2020).
Tonetti, M. S. et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 356, 911–920 (2007).
Desvarieux, M. et al. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the oral infections and vascular disease epidemiology study. J. Am. Heart Assoc. 2, e000254 (2013).
Yoneda, M. et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 12, 16 (2012).
Helenius-Hietala, J. et al. Periodontitis is associated with incident chronic liver disease — a population-based cohort study. Liver Int. 39, 583–591 (2019).
Brito, L. C. W. et al. Experimental periodontitis promotes transient vascular inflammation and endothelial dysfunction. Arch. Oral. Biol. 58, 1187–1198 (2013).
Matsuda, Y. et al. Ligature-induced periodontitis in mice induces elevated levels of circulating interleukin-6 but shows only weak effects on adipose and liver tissues. J. Periodont. Res. 51, 639–646 (2016).
O’Boyle, C. et al. Ligature-induced periodontitis induces systemic inflammation but does not alter acute outcome after stroke in mice. Int. J. Stroke 15, 175–187 (2019).
Anbinder, A. L. et al. Periodontal disease exacerbates systemic ovariectomy-induced bone loss in mice. Bone 83, 241–247 (2016).
Miyajima, S.-i. et al. Periodontitis-activated monocytes/macrophages cause aortic inflammation. Sci. Rep. 4, 5171 (2014).
Hasturk, H. et al. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscl. Thromb. Vasc. Biol. 35, 1123–1133 (2015).
Tian, J. et al. Porphyromonas gingivalis induces insulin resistance by increasing BCAA levels in mice. J. Dent. Res. 99, 839–846 (2020).
White, P. J. & Newgard, C. B. Branched-chain amino acids in disease. Science 363, 582–583 (2019).
Chavakis, T. et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J. Exp. Med. 198, 1507–1515 (2003).
Ruiz, H. H., Ramasamy, R. & Schmidt, A. M. Advanced glycation end products: building on the concept of the “Common Soil” in metabolic disease. Endocrinology 161, 1–10 (2020).
Lalla, E. et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J. Clin. Invest. 105, 1117–1124 (2000).
Nakahara, T. et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 53, 269–280 (2018).
Nagasaki, A. et al. Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation. Sci. Rep. 10, 4134 (2020).
Vasconcelos, D. F. P. et al. Decrease of pericytes is associated with liver disease caused by ligature-induced periodontitis in rats. J. Periodontol. 88, e49–e57 (2017).
Tsukasaki, M. et al. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 9, 701 (2018).
Komazaki, R. et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 7, 13950 (2017).
King, K. Y. & Goodell, M. A. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 11, 685 (2011).
Yamamoto, R. et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112–1126 (2013).
Tsukasaki, M. & Takayanagi, H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 19, 626–642 (2019).
Herrera, B. S. et al. Peripheral blood mononuclear phagocytes from patients with chronic periodontitis are primed for osteoclast formation. J. Periodontol. 85, e72–e81 (2014).
Manz, M. G. & Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 14, 302–314 (2014).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100 (2018).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161 (2018). This study shows that trained immunity can be initiated in the bone marrow through long-lasting metabolic and transcriptional adaptations in HSPCs that lead to enhanced myelopoiesis.
Pietras, E. M. et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 18, 607–618 (2016).
Fifer, K. M. et al. Positron emission tomography measurement of periodontal 18F-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J. Am. Coll. Cardiol. 57, 971–976 (2011).
Ling, M. R., Chapple, I. L. & Matthews, J. B. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 21, 714–725 (2015).
Radvar, M., Tavakkol-Afshari, J., Bajestan, M. N., Naseh, M. R. & Arab, H. R. The effect of periodontal treatment on IL-6 production of peripheral blood monocytes in aggressive periodontitis and chronic periodontitis patients. Iran. J. Immunol. 5, 100–106 (2008).
Kleinnijenhuis, J. et al. Bacille Calmette–Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334 (2020). This study shows that BCG vaccination in humans induces sustained transcriptomic myeloid bias in HSPCs associated with long-term heightened responsiveness of circulating myeloid cells to inflammatory stimuli, thereby providing human relevance for the findings of Mitroulis et al. (2018).
Bernelot Moens, S. J. et al. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res. Ther. 18, 115 (2016).
Schloss, M. J., Swirski, F. K. & Nahrendorf, M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ. Res. 126, 1242–1259 (2020).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175 (2018).
Bekkering, S. et al. Trained immunity: linking obesity and cardiovascular disease across the life-course? Trends Endocrinol. Metab. 31, 378–389 (2020).
Pink, C. et al. Longitudinal effects of systemic inflammation markers on periodontitis. J. Clin. Periodontol. 42, 988–997 (2015).
Wright, H. J., Matthews, J. B., Chapple, I. L., Ling-Mountford, N. & Cooper, P. R. Periodontitis associates with a type 1 IFN signature in peripheral blood neutrophils. J. Immunol. 181, 5775–5784 (2008).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183, 771–785 (2020).
Rafferty, B. et al. Impact of monocytic cells on recovery of uncultivable bacteria from atherosclerotic lesions. J. Intern. Med. 270, 273–280 (2011).
Kozarov, E. V., Dorn, B. R., Shelburne, C. E., Dunn, W. A. Jr & Progulske-Fox, A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 25, e17–e18 (2005).
Carrion, J. et al. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 189, 3178–3187 (2012).
Gevers, D. et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 (2014).
Schirmer, M. et al. Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe 24, 600–610.e604 (2018). Gevers et al. and Schirmer et al. demonstrate that the dominant species with increased abundance in paediatric patients with IBD are actually derived from the oral cavity instead of being typical intestinal bacteria.
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759 (2018).
Gimbrone, M. A. Jr & García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Mougeot, J. C. et al. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J. Oral Microbiol. 9, 1281562 (2017).
Dioguardi, M. et al. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer’s disease: a systematic review. J. Clin. Med. 9, 495 (2020).
Beydoun, M. A. et al. Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer’s disease dementia in a large national survey. J. Alzheimers Dis. 75, 157–172 (2020).
Kovacech, B. & Novak, M. Tau truncation is a productive posttranslational modification of neurofibrillary degeneration in Alzheimer’s disease. Curr. Alzheimer Res. 7, 708–716 (2010).
Long, J. M. & Holtzman, D. M. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339 (2019).
Muñoz, S. S., Garner, B. & Ooi, L. Understanding the role of ApoE fragments in Alzheimer’s disease. Neurochem. Res. 44, 1297–1305 (2019).
Lönn, J. et al. Lipoprotein modifications by gingipains of Porphyromonas gingivalis. J. Periodont. Res. 53, 403–413 (2018).
Poole, S. et al. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE–/– mice brains. J. Alzheimers Dis. 43, 67–80 (2015).
Hajishengallis, G., Reis, E. S., Mastellos, D. C., Ricklin, D. & Lambris, J. D. Novel mechanisms and functions of complement. Nat. Immunol. 18, 1288–1298 (2017).
Yin, C. et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat. Med. 25, 496–506 (2019).
Kantarci, A. et al. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 300, 111–120 (2018).
Kantarci, A. et al. Microglial response to experimental periodontitis in a murine model of Alzheimer’s disease. Sci. Rep. 10, 18561 (2020).
Papageorgiou, S. N. et al. Inflammatory bowel disease and oral health: systematic review and a meta-analysis. J. Clin. Periodontol. 44, 382–393 (2017).
Pietropaoli, D. et al. Occurrence of spontaneous periodontal disease in the SAMP1/YitFc Murine model of Crohn disease. J. Periodontol. 85, 1799–1805 (2014).
Moran, C. J. et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm. Bowel Dis. 19, 115–123 (2013).
Schmidt, T. S. et al. Extensive transmission of microbes along the gastrointestinal tract. eLife 8, e42693 (2019).
Yachida, S. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976 (2019).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25, 667–678 (2019).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Castellarin, M. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306 (2012).
Kostic, A. D. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298 (2012).
Figuero, E., Han, Y. W. & Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: mechanisms. Periodontol. 2000 83, 175–188 (2020).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Casasanta, M. A. et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal. 13, eaba9157 (2020). This study identifies a new mechanism linking F. nucleatum to CRC by showing that this oral bacterium promotes chemokine secretion that stimulates tumour cell migration and invasion.
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563 (2017). This paper shows that F. nucleatum adversely affects the CRC chemotherapeutic response by modulating autophagy.
Komiya, Y. et al. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 68, 1335–1337 (2019).
Abed, J. et al. Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell Infect. Microbiol. 10, 400 (2020).
Ning, Y. & Lenz, H. J. Targeting IL-8 in colorectal cancer. Expert. Opin. Ther. Targets 16, 491–497 (2012).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator annexin A1. EMBO Rep 20, e47638 (2019).
Sun, J. et al. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 9, 6306–6321 (2020).
Hashimoto, M. et al. Periodontitis and Porphyromonas gingivalis in preclinical stage of arthritis patients. PLoS ONE 10, e0122121 (2015).
Mikuls, T. R. et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 1090–1100 (2014).
Al-Katma, M. K., Bissada, N. F., Bordeaux, J. M., Sue, J. & Askari, A. D. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J. Clin. Rheumatol. 13, 134–137 (2007).
Khare, N. et al. Nonsurgical periodontal therapy decreases the severity of rheumatoid arthritis: a case-control study. J. Contemp. Dent. Pract. 17, 484–488 (2016).
Ortiz, P. et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J. Periodontol. 80, 535–540 (2009).
Maresz, K. J. et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9, e1003627 (2013).
Gully, N. et al. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS ONE 9, e100838 (2014). Maresz et al. is the first study to mechanistically link a periodontal pathogen (P. gingivalis) with generation of citrullinated autoantigens that are involved in the pathogenesis of rheumatoid arthritis. Gully et al. provide independent confirmation that peptidylarginine deiminase of P. gingivalis promotes the induction of ACPAs and experimental arthritis.
Wegner, N. et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheumatol. 62, 2662–2672 (2010).
Lübcke, P. M. et al. Periodontal treatment prevents arthritis in mice and methotrexate ameliorates periodontal bone loss. Sci. Rep. 9, 8128 (2019).
Flak, M. B. et al. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight 4, e125191 (2019).
Boyaka, P. N. & Fujihashi, K. in Clinical Immunology (Fifth Edition) (eds Rich, R. R. et al.) 285–298 (Elsevier, 2019).
Gill, N., Wlodarska, M. & Finlay, B. B. The future of mucosal immunology: studying an integrated system-wide organ. Nat. Immunol. 11, 558–560 (2010).
Scribano, M. L. Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J. Gastroenterol. 24, 2457–2467 (2018).
Calderón-Gómez, E. et al. Commensal-specific CD4+ cells from patients with Crohn’s disease have a T-helper 17 inflammatory profile. Gastroenterology 151, 489–500 (2016).
Wang, J. et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell–dependent inflammation. J. Exp. Med. 211, 2397–2410 (2014).
D’Amico, F., Baumgart, D. C., Danese, S. & Peyrin-Biroulet, L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin. Gastroenterol. Hepatol. 18, 1663–1672 (2020).
Miles, B. et al. Secondary lymphoid organ homing phenotype of human myeloid dendritic cells disrupted by an intracellular oral pathogen. Infect. Immun. 82, 101–111 (2014).
Sainz, J. & Sata, M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler. Thromb. Vasc. Biol. 27, 263–265 (2007).
Rajendran, M. et al. Systemic antibiotic therapy reduces circulating inflammatory dendritic cells and Treg-Th17 plasticity in periodontitis. J. Immunol. 202, 2690–2699 (2019).
Chen, M. et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 311, 1160–1164 (2006).
Hajishengallis, G., Chavakis, T. & Lambris, J. D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000 84, 14–34 (2020).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017). This study provides causal evidence linking CHIP to atherosclerosis. The authors show that atherosclerosis progresses more rapidly in mice transplanted with TET2-deficient bone marrow cells, in great part attributed to the increased inflammatory activity of TET2-deficient macrophage progeny.
Eskan, M. A. et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 13, 465–473 (2012).
Shin, J. et al. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 7, 307ra155 (2015).
Kourtzelis, I. et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 20, 40–49 (2019).
Li, X. et al. The DEL-1/β3 integrin axis promotes regulatory T cell responses during inflammation resolution. J. Clin. Invest. 130, 6261–6277 (2020).
Mitroulis, I. et al. Secreted protein Del-1 regulates myelopoiesis in the hematopoietic stem cell niche. J. Clin. Invest. 127, 3624–3639 (2017).
Adler, C. J. et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 45, 450–455 (2013).
Peres, M. A. et al. Oral diseases: a global public health challenge. Lancet 394, 249–260 (2019).
Dutzan, N. et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 10, eaat0797 (2018).
Abe, T. & Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 394, 49–54 (2013).
Hajishengallis, G. et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506 (2011).
Payne, M. A. et al. Horizontal and vertical transfer of oral microbial dysbiosis and periodontal disease. J. Dent. Res. 98, 1503–1510 (2019).
Penkov, S., Mitroulis, I., Hajishengallis, G. & Chavakis, T. Immunometabolic crosstalk: an ancestral principle of trained immunity? Trends Immunol. 40, 1–11 (2019).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 (2018).
Nahrendorf, M. Myeloid cell contributions to cardiovascular health and disease. Nat. Med. 24, 711–720 (2018).
Murphy, A. J. & Tall, A. R. Disordered haematopoiesis and athero-thrombosis. Eur. Heart J. 37, 1113–1121 (2016).
Barrett, T. J., Murphy, A. J., Goldberg, I. J. & Fisher, E. A. Diabetes-mediated myelopoiesis and the relationship to cardiovascular risk. Ann. N. Y. Acad. Sci. 1402, 31–42 (2017).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Acknowledgements
The authors’ research is supported by grants from the US National Institutes of Health (DE024153, DE024716, DE029436 to G.H.; DE026152 and DE028561 to G.H. and T.C.) and the German Research Foundation (CRC-TR127 and CRC1181 to T.C.). The authors regret that several important studies could only be cited indirectly through comprehensive reviews, owing to space and reference number limitations.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks N. Kamada, T. Van Dyke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Inflammatory bowel disease
-
(IBD). A chronic inflammatory disorder of the gastrointestinal tract with a complex aetiology, including genetic, environmental, microbial and host immune factors. Two major IBD conditions are Crohn’s disease (which may affect anywhere in the digestive tract) and ulcerative colitis (which affects only the colon).
- Microbiome/microbiota
-
Microbiota is a diverse microbial community that exists within a defined anatomical niche (for example, an environmentally exposed surface of a mammalian organism). The term microbiome represents the microbial community, its combined genetic material and its collective functions.
- Dysbiosis
-
An imbalanced interaction, between bacteria in a community and/or between the microbial community and the host immune system, which has detrimental effects on the host (as in periodontitis or inflammatory bowel disease). The microbial imbalance derives from changes in the abundance and/or influence of individual species relative to their abundance or influence in health.
- Periodontal treatment
-
A procedure that involves mechanical debridement (scaling and root planing) to remove the microbial biofilm (dental plaque) and calculus (tartar) from the tooth surfaces and beneath the gingiva to enable inflammation resolution, and to smooth the root surfaces to deter further biofilm/calculus build-up. Advanced periodontitis may additionally require periodontal surgery, such as pocket reduction surgery, to reduce the depth of the pockets between the teeth and the surrounding gingiva.
- Inflammophilic
-
From inflammation and the Greek suffix philic indicating fondness. A property of bacteria that thrive under inflammatory conditions by utilizing products derived from the inflammatory breakdown of tissues as nutritional substrates.
- Periodontal pockets
-
The physiological narrow space between the tooth root and the free gingiva is known as subgingival crevice; during periodontitis progression, however, this crevice deepens into a periodontal pocket, which is a pathognomonic feature of the disease.
- Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis
-
(NAFLD/NASH). NAFLD is a condition associated with obesity and metabolic syndrome and involves excessive fat accumulation (steatosis) in the liver in the absence of significant alcohol consumption. NAFLD may progress to NASH, where fat accumulation in the liver is accompanied by inflammation, leading to fibrosis and ultimately cirrhosis (advanced scarring/fibrosis) and end-stage liver failure.
- HbA1c
-
HbA1c, or glycated haemoglobin, represents a form of haemoglobin that has glucose attached to it via a non-enzymatic reaction called glycation. The concentration of HbA1c in blood reflects the average levels of blood glucose of the previous 3–4 months. Therefore, HbA1c is used not only in the diagnosis of diabetes but, importantly, as a measure for monitoring glycaemic control in patients with diabetes.
- Atheromatous plaque
-
Fat accumulation in the intima (inner lining) of arteries that results in fibrous thickening or calcification of the arteries, restriction of blood flow and an enhanced risk of thrombotic occlusion.
- Emergency myelopoiesis
-
A tightly regulated process initiated in response to systemic infection for de novo generation of mature myeloid cells, resulting from accelerated proliferation and enhanced myeloid differentiation of progenitors in the bone marrow. The goal is to meet the increased demand for neutrophils and other phagocytes that are consumed in large quantities during systemic infections.
- Rheumatoid arthritis
-
A chronic inflammatory autoimmune disease typified by production of autoantibodies to various targets (for example, citrullinated matrix proteins) and induction of inflammation that causes progressive erosion of cartilage and bone in the joints. Its aetiology is complex and includes both genetic and environmental factors.
- Keystone pathogen
-
A pathogen that exerts a disproportionately large effect on its community relative to its abundance, forming the ‘keystone’ of the community’s structure; for instance, in experimental periodontitis, low-abundance Porphyromonas gingivalis modulates the size and composition of the local microbial community in a manner that promotes dysbiosis.
- Gingipains
-
A family of Porphyromonas gingivalis-derived trypsin-like cysteine proteases that make a major contribution to its virulence. High-molecular mass arginine-specific gingipain A (HRgpA), arginine-specific gingipain B (RgpB) and lysine-specific gingipain (Kgp) are the members of the gingipain family.
- Alzheimer disease
-
Progressive neuroinflammatory and neurodegenerative brain disease, characterized by extraneuronal deposition of amyloid-β peptides (neuritic plaques) and intraneuronal accumulation of hyperphosphorylated and fragmented tau, a microtubule-associated protein (neurofibrillary tangles).
- Pathobionts
-
Organisms that are generally benign or commensal but that have the capacity to promote pathology under specific conditions of disrupted host–microbiota homeostasis (for example, resulting from immune deficiencies, antibiotic treatment, tissue damage or dietary shifts).
- Non-canonical inflammasome
-
Inflammasome is a cytosolic, multiprotein complex that activates caspases (predominantly caspase 1) in response to infection or injury. This in turn results in cleavage and secretion of inflammatory cytokines, such as IL-1β and IL-18. Non-canonical is a caspase 1-independent but caspase 11-mediated pathway of inflammasome activation, which is crucial for controlling Gram-negative bacterial infections and can also trigger caspase 1 activation and subsequent maturation and secretion of IL-1β and IL-18. Both pathways may induce a specific form of cell death, called pyroptosis.
- Citrullination
-
Post-translational modification of a protein involving deimination of arginine by peptidylarginine deiminase to generate citrulline. Uncontrolled citrullination may result in neoepitopes that induce autoantibody generation.
- Clonal haematopoiesis of indeterminate potential
-
(CHIP). Age-related acquisition of somatic mutations, which confer haematopoietic stem cell clonal expansion advantage and are associated with increased risk of myeloid malignancies, cardiovascular disease and all-cause mortality. The generated mutant myeloid cells comprise a disproportionately large fraction of leukocytes (in peripheral blood and tissues) and display a hyper-inflammatory phenotype, suggesting that CHIP may also be related to chronic inflammatory diseases in general.
Rights and permissions
About this article
Cite this article
Hajishengallis, G., Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 21, 426–440 (2021). https://doi.org/10.1038/s41577-020-00488-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-020-00488-6
This article is cited by
-
Causal association between periodontitis and systemic diseases: a systematic review and meta-analysis of mendelian randomization studies
BMC Oral Health (2026)
-
Targeted repair of oral mucosal injury: emerging applications of biomaterials-based drug delivery systems
Journal of Nanobiotechnology (2026)
-
Klotho protects the osteogenic function of human periodontal ligament stem cells in periodontitis by inhibiting NOX4-mediated ferroptosis
Stem Cell Research & Therapy (2026)
-
Targeted Inhibition of P. gingivalis OMV-derived TsRNA by tetrahedral framework nucleic acids promotes periodontal regeneration
Journal of Nanobiotechnology (2026)
-
Periodontal disease-associated oral and gut microbiome changes in female rheumatoid arthritis patients
BMC Oral Health (2026)