Abstract
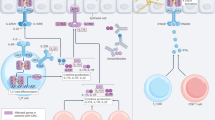
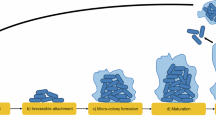
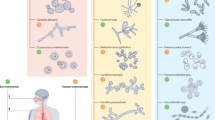
Increased use of implanted medical devices, use of immunosuppressants and an ageing population have driven the rising frequency of fungal biofilm-related diseases. Fungi are now recognized by the World Health Organization (WHO) as an emergent threat to human health, with most medically important species defined as critical or high-priority organisms capable of forming biofilms. Although we strive for a better understanding of diagnostic and therapeutic approaches to detect and treat these fungal diseases more generally, the issue of hard-to-treat biofilms is an ever-increasing problem. These are communities of interspersed cells that are attached to one another on a surface, such as a catheter, or trapped into a cavity such as a paranasal sinus. Biofilms are difficult to detect, difficult to remove and intrinsically tolerant to most antifungal agents. These factors can lead to devastating consequences for the patient, including unnecessary morbidity and mortality, need for reoperations and prolonged hospital stay. This Review describes the breadth and growing impact fungal biofilms have on patient management and explains the mechanisms promoting biofilm formation, focusing on how targeting these can improve therapeutic options.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Camara, M. et al. Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 8, 42 (2022).
Marrie, T. J. & Costerton, J. W. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J. Clin. Microbiol. 19, 687–693 (1984).
Denning, D. W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24, e428–e438 (2024). This work presents a comprehensive literature analysis of an updated global burden of fungal diseases.
World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. WHO https://www.who.int/publications/i/item/9789240060241 (2022).
Ramage, G. et al. Our current clinical understanding of Candida biofilms: where are we two decades on? APMIS 131, 636–653 (2023).
Donlan, R. M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8, 881–890 (2002).
Rumbaugh, K. P. & Bjarnsholt, T. Microbial primer: in vivo biofilm. Microbiology 169, 001407 (2023).
Nett, J. et al. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51, 510–520 (2007). This pioneering study identifies the function for β-glucans in conferring biofilm tolerance in the ECM.
Sauer, K. et al. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 20, 608–620 (2022).
Williams, C. & Ramage, G. Fungal biofilms in human disease. Adv. Exp. Med. Biol. 831, 11–27 (2015).
Robey, A. B. et al. The changing face of paranasal sinus fungus balls. Ann. Otol. Rhinol. Laryngol. 118, 500–505 (2009).
Nguyen, U. T. & Kalan, L. R. Forgotten fungi: the importance of the skin mycobiome. Curr. Opin. Microbiol. 70, 102235 (2022).
Kalan, L. & Grice, E. A. Fungi in the wound microbiome. Adv. Wound Care 7, 247–255 (2018).
Benedict, K., Jackson, B. R., Chiller, T. & Beer, K. D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. 68, 1791–1797 (2019).
Ramage, G. et al. in Antibiofilm Strategies: Current and Future Applications to Prevent, Control and Eradicate Biofilms (eds Richter, K. & Kragh, K. N.) 441–465 (Springer, 2022).
Coco, B. J. et al. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral. Microbiol. Immunol. 23, 377–383 (2008).
Parahitiyawa, N. B. et al. Interspecies variation in Candida biofilm formation studied using the Calgary biofilm device. APMIS 114, 298–306 (2006).
Nett, J. E., Cain, M. T., Crawford, K. & Andes, D. R. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J. Clin. Microbiol. 49, 1426–1433 (2011).
Budtz-Jorgensen, E. The significance of Candida albicans in denture stomatitis. Scand. J. Dent. Res. 82, 151–190 (1974).
Ramage, G., Tomsett, K., Wickes, B. L., Lopez-Ribot, J. L. & Redding, S. W. Denture stomatitis: a role for Candida biofilms. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 98, 53–59 (2004).
Fujinami, W., Nishikawa, K., Ozawa, S., Hasegawa, Y. & Takebe, J. Correlation between the relative abundance of oral bacteria and Candida albicans in denture and dental plaques. J. Oral. Biosci. 63, 175–183 (2021).
O’Donnell, L. E. et al. The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS ONE 10, e0137717 (2015).
Redfern, J. et al. The denture microbiome in health and disease: an exploration of a unique community. Lett. Appl. Microbiol. 75, 195–209 (2022).
Eidt, G., Waltermann, E. D. M., Hilgert, J. B. & Arthur, R. A. Candida and dental caries in children, adolescents and adults: a systematic review and meta-analysis. Arch. Oral. Biol. 119, 104876 (2020).
Slazhneva, E. et al. Candida species detection in patients with chronic periodontitis: a systematic review and meta-analysis. Clin. Exp. Dent. Res. 8, 1354–1375 (2022).
Delliere, S. et al. Analysis of microbiota and mycobiota in fungal ball rhinosinusitis: specific interaction between Aspergillus fumigatus and Haemophilus influenza? J. Fungi. https://doi.org/10.3390/jof7070550 (2021).
Hashemi, S. J. et al. A case of fungus ball-type pansinusitis due to Fusarium proliferatum. Mycopathologia 180, 251–255 (2015).
Lee, J. T. et al. Fungal and bacterial microbiome in sinus mucosa of patients with and without chronic rhinosinusitis. Laryngoscope 134, 1054–1062 (2024).
Nambiar, M. et al. Mycotic infections—mucormycosis and oral candidiasis associated with COVID-19: a significant and challenging association. J. Oral. Microbiol. 13, 1967699 (2021).
Roh, D. et al. Sinonasal microbiome and inflammatory profiles in fungal ball and chronic rhinosinusitis. Auris Nasus Larynx 51, 242–250 (2024).
Martin, T. J., Kerschner, J. E. & Flanary, V. A. Fungal causes of otitis externa and tympanostomy tube otorrhea. Int. J. Pediatr. Otorhinolaryngol. 69, 1503–1508 (2005).
Zhang, X., Sun, X., Wang, Z., Zhang, Y. & Hou, W. Keratitis-associated fungi form biofilms with reduced antifungal drug susceptibility. Invest. Ophthalmol. Vis. Sci. 53, 7774–7778 (2012).
Muni, I., Behera, H. S., Sahu, S. K., Priyadarshini, S. R. & Das, S. Microbiological profile of culture-positive fungal keratitis. Eye Contact Lens https://doi.org/10.1097/ICL.0000000000001089 (2024).
Abdulkareem, A. F., Lee, H. H., Ahmadi, M. & Martinez, L. R. Fungal serotype-specific differences in bacterial–yeast interactions. Virulence 6, 652–657 (2015).
Aslanyan, L. et al. The crucial role of biofilms in Cryptococcus neoformans survival within macrophages and colonization of the central nervous system. J. Fungi. https://doi.org/10.3390/jof3010010 (2017).
Kolpen, M. et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 77, 1015–1022 (2022).
Hong, G. et al. Distinct community structures of the fungal microbiome and respiratory health in adults with cystic fibrosis. J. Cyst. Fibros. 22, 636–643 (2023).
Cuthbertson, L. et al. The fungal airway microbiome in cystic fibrosis and non-cystic fibrosis bronchiectasis. J. Cyst. Fibros. 20, 295–302 (2021).
Amin, R., Dupuis, A., Aaron, S. D. & Ratjen, F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 137, 171–176 (2010).
Kean, R. et al. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front. Microbiol. 8, 258 (2017).
Sobel, J. D. Editorial commentary: Vaginal biofilm: much ado about nothing, or a new therapeutic challenge? Clin. Infect. Dis. 61, 607–608 (2015).
Wu, X. et al. Biofilm formation of Candida albicans facilitates fungal infiltration and persister cell formation in vaginal candidiasis. Front Microbiol 11, 1117 (2020).
McKloud, E. et al. Recurrent vulvovaginal candidiasis: a dynamic interkingdom biofilm disease of Candida and Lactobacillus. mSystems 6, e0062221 (2021).
Pan, Y. et al. Candida causes recurrent vulvovaginal candidiasis by forming morphologically disparate biofilms on the human vaginal epithelium. Biofilm 6, 100162 (2023). This paper elegantly demonstrates that Candida is able to form biofilms on vaginal mucosa, a continued area of contention that impacts antifungal management.
Bumroongthai, K., Chetanachan, P., Niyomtham, W., Yurayart, C. & Prapasarakul, N. Biofilm production and antifungal susceptibility of co-cultured Malassezia pachydermatis and Candida parapsilosis isolated from canine seborrheic dermatitis. Med Mycol. 54, 544–549 (2016).
Markantonatou, A. M., Samaras, K. & Vyzantiadis, T. A. Dermatophytic biofilms: characteristics, significance and treatment approaches. J. Fungi. https://doi.org/10.3390/jof9020228 (2023).
Chellan, G. et al. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J. Clin. Microbiol. 48, 2097–2102 (2010).
Dowd, S. E. et al. Survey of fungi and yeast in polymicrobial infections in chronic wounds. J. Wound Care 20, 40–47 (2011).
Short, B. et al. in Current Clinical Microbiology Reports Vol. 10 (ed. Diezmann, S.) 9–16 (Springer Link, 2023).
Bharti, S., Zakir, F., Mirza, M. A. & Aggarwal, G. Antifungal biofilm strategies: a less explored area in wound management. Curr. Pharm. Biotechnol. 23, 1497–1513 (2022).
Sansom, S. E. et al. Rapid environmental contamination with Candida auris and multidrug-resistant bacterial pathogens near colonized patients. Clin. Infect. Dis. 78, 1276–1284 (2024).
Arciola, C. R., Campoccia, D. & Montanaro, L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 16, 397–409 (2018).
O’Donnell, L. E. et al. Dentures are a reservoir for respiratory pathogens. J. Prosthodont. 25, 99–104 (2016).
van Charante, F. et al. Microbial diversity and antimicrobial susceptibility in endotracheal tube biofilms recovered from mechanically ventilated COVID-19 patients. Biofilm 4, 100079 (2022).
Baidya, S. et al. Biofilm formation by pathogens causing ventilator-associated pneumonia at intensive care units in a tertiary care hospital: an armor for refuge. Biomed. Res Int 2021, 8817700 (2021).
Velasquez-Garcia, L., Mejia-Sanjuanelo, A., Viasus, D. & Carratala, J. Causative agents of ventilator-associated pneumonia and resistance to antibiotics in COVID-19 patients: a systematic review. Biomedicines https://doi.org/10.3390/biomedicines10061226 (2022).
Cetinkaya, E. et al. Simultaneous chronic invasive fungal infection and tracheal fungus ball mimicking cancer in an immunocompetent patient. Case Rep. Med. 2016, 2416452 (2016).
McGinniss, J. E. et al. Molecular analysis of the endobronchial stent microbial biofilm reveals bacterial communities that associate with stent material and frequent fungal constituents. PLoS ONE 14, e0217306 (2019).
Huang, D., Qi, M., Hu, Y., Yu, M. & Liang, Z. The impact of Candida spp airway colonization on clinical outcomes in patients with ventilator-associated pneumonia: a systematic review and meta-analysis. Am. J. Infect. Control 48, 695–701 (2020).
Durliat, A., Locatelli-Sanchez, M., Wallet, F. & Allaouchiche, B. Tracheal stent aspergillosis occurring after aortic allograft of the trachea. Transpl. Infect. Dis. 24, e13965 (2022).
Tumbarello, M. et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45, 1843–1850 (2007).
Walsh, T. J., Schlegel, R., Moody, M. M., Costerton, J. W. & Salcman, M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery 18, 373–375 (1986).
Rajendran, R. et al. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin. Microbiol. Infect. 22, 87–93 (2016). This paper is one of the first to demonstrate that different levels of biofilm formation correlate with clinical outcomes.
Rajendran, R. et al. A prospective surveillance study of candidaemia: epidemiology, risk factors, antifungal treatment and outcome in hospitalized patients. Front Microbiol 7, 915 (2016).
Pappas, P. G. et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, e1–e50 (2016).
McGhee, W., Michaels, M. G., Martin, J. M., Mazariegos, G. V. & Green, M. Antifungal lock therapy with liposomal amphotericin B: a prospective trial. J. Pediatr. Infect. Dis. Soc. 5, 80–84 (2016).
Paul DiMondi, V., Townsend, M. L., Johnson, M. & Durkin, M. Antifungal catheter lock therapy for the management of a persistent Candida albicans bloodstream infection in an adult receiving hemodialysis. Pharmacotherapy 34, e120–e127 (2014).
Walraven, C. J. & Lee, S. A. Antifungal lock therapy. Antimicrob. Agents Chemother. 57, 1–8 (2013).
Di Benedetto, G. et al. Giant Candida mycetoma in an ascending aorta tubular graft. J. Card. Surg. 28, 557–560 (2013).
Hebert, J., Barr, E. & Magee, C. Pacemaker-related Candida parapsilosis fungaemia in an immunosuppressed renal transplant recipient. BMJ Case Rep. https://doi.org/10.1136/bcr-2021-242917 (2021).
Herndon, C. L. et al. Treatment outcomes of fungal periprosthetic joint infection. J. Arthroplast. 38, 2436–2440.e1 (2023).
Diop, S. et al. Biofilm assessment and metagenomic analysis of venoarterial extracorporeal membrane oxygenation cannulas and membrane oxygenators. ASAIO J. 70, 199–206 (2024).
Eyre, D. W. et al. A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 379, 1322–1331 (2018). This paper demonstrates that C. auris is capable of persisting on surfaces outwith the human body to aid transmission.
Biswal, M. et al. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J. Hosp. Infect. 97, 363–370 (2017).
Weber, D. J., Rutala, W. A., Anderson, D. J. & Sickbert-Bennett, E. E. Biofilms on medical instruments and surfaces: do they interfere with instrument reprocessing and surface disinfection. Am. J. Infect. Control. 51, A114–A119 (2023).
Lohse, M. B., Gulati, M., Johnson, A. D. & Nobile, C. J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 16, 19–31 (2018).
Ajesh, K. & Sreejith, K. Cryptococcus laurentii biofilms: structure, development and antifungal drug resistance. Mycopathologia 174, 409–419 (2012).
Fox, E. P. et al. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 96, 1226–1239 (2015).
Harding, M. W., Marques, L. L., Howard, R. J. & Olson, M. E. Can filamentous fungi form biofilms? Trends Microbiol. 17, 475–480 (2009).
Kean, R. et al. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere https://doi.org/10.1128/mSphere.00334-18 (2018).
Laffey, S. F. & Butler, G. Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology 151, 1073–1081 (2005).
Lopes, W. et al. Geometrical distribution of Cryptococcus neoformans mediates flower-like biofilm development. Front. Microbiol. 8, 2534 (2017). This paper elegantly shows that C. neoformans is capable of forming defined biofilm structures that aid development and dispersal.
Malinovska, Z., Conkova, E. & Vaczi, P. Biofilm formation in medically important Candida species. J. Fungi. https://doi.org/10.3390/jof9100955 (2023).
Morelli, K. A., Kerkaert, J. D. & Cramer, R. A. Aspergillus fumigatus biofilms: toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 17, e1009794 (2021).
Ravi, S., Pierce, C., Witt, C. & Wormley, F. L. Jr. Biofilm formation by Cryptococcus neoformans under distinct environmental conditions. Mycopathologia 167, 307–314 (2009).
Scherer, A. K. et al. Redundant Trojan horse and endothelial-circulatory mechanisms for host-mediated spread of Candida albicans yeast. PLoS Pathog. 16, e1008414 (2020).
Sephton-Clark, P. C. S. & Voelz, K. Spore germination of pathogenic filamentous fungi. Adv. Appl. Microbiol. 102, 117–157 (2018).
Silva, S. et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 36, 288–305 (2012).
Wang, T.W. et al. Functional redundancy in Candida auris cell surface adhesins crucial for cell-cell interaction and aggregation. Nat. Commun. 15, 9212 (2024).
Cota, E. & Hoyer, L. L. The Candida albicans agglutinin-like sequence family of adhesins: functional insights gained from structural analysis. Future Microbiol. 10, 1635–1548 (2015).
Dutton, L. C. et al. Transcriptional landscape of trans-kingdom communication between Candida albicans and Streptococcus gordonii. Mol. Oral. Microbiol. 31, 136–161 (2016). This study demonstrates the specific adhesion and interaction between yeasts and bacteria, indicating common partnerships in complex environments.
Sundstrom, P. Adhesion in Candida spp. Cell Microbiol. 4, 461–469 (2002).
Golan, N., Schwartz-Perov, S., Landau, M. & Lipke, P. N. Structure and conservation of amyloid spines from the Candida albicans Als5 adhesin. Front. Mol. Biosci. 9, 926959 (2022).
Lipke, P. N., Klotz, S. A., Dufrene, Y. F., Jackson, D. N. & Garcia-Sherman, M. C. Amyloid-like β-aggregates as force-sensitive switches in fungal biofilms and infections. Microbiol. Mol. Biol. Rev. https://doi.org/10.1128/MMBR.00035-17 (2018).
Timmermans, B., De Las Penas, A., Castano, I. & Van Dijck, P. Adhesins in Candida glabrata. J. Fungi. https://doi.org/10.3390/jof4020060 (2018).
Dague, E., Alsteens, D., Latge, J. P. & Dufrene, Y. F. High-resolution cell surface dynamics of germinating Aspergillus fumigatus conidia. Biophys. J. 94, 656–660 (2008).
Upadhyay, S. K. et al. Identification and characterization of a laminin-binding protein of Aspergillus fumigatus: extracellular thaumatin domain protein (AfCalAp). J. Med. Microbiol. 58, 714–722 (2009).
Liu, H. et al. Aspergillus fumigatus CalA binds to integrin ɑ5β1 and mediates host cell invasion. Nat. Microbiol. 2, 16211 (2016).
Martinez, L. R. & Casadevall, A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 73, 6350–6362 (2005).
Gonzalez-Ramirez, A. I., Ramirez-Granillo, A., Medina-Canales, M. G., Rodriguez-Tovar, A. V. & Martinez-Rivera, M. A. Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol. 16, 243 (2016).
Nobile, C. J. et al. Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 18, 1017–1024 (2008). This study identifies the first core regulatory transcriptional network in C. albicans.
Bing, J. et al. Clinical isolates of Candida auris with enhanced adherence and biofilm formation due to genomic amplification of ALS4. PLoS Pathog. 19, e1011239 (2023).
Santana, D. J. et al. A Candida auris-specific adhesin, Scf1, governs surface association, colonization, and virulence. Science 381, 1461–1467 (2023). This study categorizes the function of a novel C. auris specific adhesin, Scf1, in relation to colonization, biofilm formation and virulence.
Martinez, L. R. & Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50, 1021–1033 (2006).
Kowalski, C. H. et al. Fungal biofilm morphology impacts hypoxia fitness and disease progression. Nat. Microbiol. 4, 2430–2441 (2019).
Kowalski, C. H., Morelli, K. A., Stajich, J. E., Nadell, C. D. & Cramer, R. A. A heterogeneously expressed gene family modulates the biofilm architecture and hypoxic growth of Aspergillus fumigatus. mBio https://doi.org/10.1128/mBio.03579-20 (2021).
Pentland, D. R., Davis, J., Muhlschlegel, F. A. & Gourlay, C. W. CO2 enhances the formation, nutrient scavenging and drug resistance properties of C. albicans biofilms. NPJ Biofilms Microbiomes 7, 67 (2021).
Rossignol, T. et al. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot. Cell 8, 550–559 (2009).
Pierce, C. G. et al. The Candida albicans biofilm matrix: composition, structure and function. J. Fungi. https://doi.org/10.3390/jof3010014 (2017).
Zarnowski, R. et al. A common vesicle proteome drives fungal biofilm development. Proc. Natl Acad. Sci. USA 119, e2211424119 (2022). This study identifies a conserved component across the extracellular vesicle proteome of biofilms formed by various Candida spp.
Kong, E. F. et al. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 7, e01365-16 (2016).
Uppuluri, P. et al. Candida albicans dispersed cells are developmentally distinct from biofilm and planktonic cells. mBio https://doi.org/10.1128/mBio.01338-18 (2018).
Uppuluri, P. et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 6, e1000828 (2010).
Nobile, C. J. & Mitchell, A. P. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 8, 1382–1391 (2006).
Kumamoto, C. A. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc. Natl Acad. Sci. USA 102, 5576–5581 (2005).
Blankenship, J. R. & Mitchell, A. P. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9, 588–594 (2006).
Nobile, C. J. & Mitchell, A. P. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 15, 1150–1155 (2005).
Nobile, C. J. et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148, 126–138 (2012).
Liu, S., Le Mauff, F., Sheppard, D. C. & Zhang, S. Filamentous fungal biofilms: conserved and unique aspects of extracellular matrix composition, mechanisms of drug resistance and regulatory networks in Aspergillus fumigatus. NPJ Biofilms Microbiomes 8, 83 (2022).
Subroto, E., van Neer, J., Valdes, I. & de Cock, H. Growth of Aspergillus fumigatus in biofilms in comparison to Candida albicans. J. Fungi. https://doi.org/10.3390/jof8010048 (2022).
Bom, V. L. et al. The Aspergillus fumigatus sitA phosphatase homologue is important for adhesion, cell wall integrity, biofilm formation, and virulence. Eukaryot. Cell 14, 728–744 (2015).
Lin, C. J., Hou, Y. H. & Chen, Y. L. The histone acetyltransferase GcnE regulates conidiation and biofilm formation in Aspergillus fumigatus. Med. Mycol. 58, 248–259 (2020).
Robbins, N. et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 7, e1002257 (2011).
Granger, B. L. Insight into the antiadhesive effect of yeast wall protein 1 of Candida albicans. Eukaryot. Cell 11, 795–805 (2012).
McCall, A. D., Pathirana, R. U., Prabhakar, A., Cullen, P. J. & Edgerton, M. Author correction: Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ Biofilms Microbiomes 7, 91 (2021).
Baillie, G. S. & Douglas, L. J. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46, 397–403 (2000).
Nett, J. E., Sanchez, H., Cain, M. T. & Andes, D. R. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 202, 171–175 (2010).
Rajendran, R. et al. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell 12, 420–429 (2013).
Zarnowski, R. et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. 16, e2006872 (2018).
Rizzo, J. et al. Coregulation of extracellular vesicle production and fluconazole susceptibility in Cryptococcus neoformans. mBio 14, e0087023 (2023).
Bitencourt, T. A. et al. Fungal extracellular vesicles are involved in intraspecies intracellular communication. mBio 13, e0327221 (2022).
Wuyts, J., Van Dijck, P. & Holtappels, M. Fungal persister cells: the basis for recalcitrant infections? PLoS Pathog. 14, e1007301 (2018).
Rossoni, R. D. et al. The postbiotic activity of Lactobacillus paracasei 28.4 against Candida auris. Front. Cell Infect. Microbiol. 10, 397 (2020).
Bink, A. et al. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 55, 4033–4037 (2011).
Diezmann, S., Leach, M. D. & Cowen, L. E. Functional divergence of Hsp90 genetic interactions in biofilm and planktonic cellular states. PLoS One 10, e0137947 (2015).
Denega, I., d’Enfert, C. & Bachellier-Bassi, S. Candida albicans biofilms are generally devoid of persister cells. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.01979-18 (2019).
Fux, C. A., Shirtliff, M., Stoodley, P. & Costerton, J. W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 13, 58–63 (2005).
Glazier, V. E. et al. The Candida albicans reference strain SC5314 contains a rare, dominant allele of the transcription factor Rob1 that modulates filamentation, biofilm formation, and oral commensalism. mBio 14, e0152123 (2023).
Wakade, R. S., Huang, M., Mitchell, A. P., Wellington, M. & Krysan, D. J. Intravital imaging of Candida albicans identifies differential in vitro and in vivo filamentation phenotypes for transcription factor deletion mutants. mSphere 6, e0043621 (2021).
Huang, M. Y., Woolford, C. A., May, G., McManus, C. J. & Mitchell, A. P. Circuit diversification in a biofilm regulatory network. PLoS Pathog. 15, e1007787 (2019).
Cravener, M. V. et al. Reinforcement amid genetic diversity in the Candida albicans biofilm regulatory network. PLoS Pathog. 19, e1011109 (2023).
Delaney, C. et al. An integrated transcriptomic and metabolomic approach to investigate the heterogeneous Candida albicans biofilm phenotype. Biofilm 5, 100112 (2023).
Rajendran, R. et al. Integrating Candida albicans metabolism with biofilm heterogeneity by transcriptome mapping. Sci. Rep. 6, 35436 (2016).
Holland, L. M. et al. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 10, e1004365 (2014).
Ramage, G., Rajendran, R., Sherry, L. & Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 528521 (2012).
Kuhn, D. M., George, T., Chandra, J., Mukherjee, P. K. & Ghannoum, M. A. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46, 1773–1780 (2002).
Bachmann, S. P. et al. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46, 3591–3596 (2002).
Bachmann, S. P., Patterson, T. F. & Lopez-Ribot, J. L. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 40, 2228–2230 (2002).
Jacobson, M. J., Steckelberg, K. E., Piper, K. E., Steckelberg, J. M. & Patel, R. In vitro activity of micafungin against planktonic and sessile Candida albicans isolates. Antimicrob. Agents Chemother. 53, 2638–2639 (2009).
Jacobson, M. J., Piper, K. E., Nguyen, G., Steckelberg, J. M. & Patel, R. In vitro activity of anidulafungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 52, 2242–2243 (2008).
Carolus, H., Pierson, S., Lagrou, K. & Van Dijck, P. Amphotericin B and other polyenes—discovery, clinical use, mode of action and drug resistance. J. Fungi. https://doi.org/10.3390/jof6040321 (2020).
Li, P., Seneviratne, C. J., Alpi, E., Vizcaino, J. A. & Jin, L. Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters. Antimicrob. Agents Chemother. 59, 6101–6112 (2015).
Sigera, L. S. M. & Denning, D. W. Flucytosine and its clinical usage. Ther. Adv. Infect. Dis. 10, 20499361231161387 (2023).
Hoenigl, M. et al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81, 1703–1729 (2021). This comprehensive review provides a detailed overview of the new pipeline of antifungal drugs.
Neoh, C. F., Jeong, W., Kong, D. C. & Slavin, M. A. The antifungal pipeline for invasive fungal diseases: what does the future hold? Expert. Rev. Anti Infect. Ther. 21, 577–594 (2023).
Lanier, C. & Melton, T. C. Oteseconazole for the treatment of recurrent vulvovaginal candidiasis: a drug review. Ann. Pharmacother. 58, 636–644 (2024).
Michael, M. The FDA approves new antifungal oteseconazole. Chemical & Engineering News https://cen.acs.org/business/FDA-approves-new-antifungal-oteseconazole/100/i16 (2022).
Sobel, J. D. et al. Efficacy and safety of oteseconazole in recurrent vulvovaginal candidiasis. NEJM Evid. 1, EVIDoa2100055 (2022).
Wiederhold, N. P. Pharmacodynamics, mechanisms of action and resistance, and spectrum of activity of new antifungal agents. J. Fungi 8, 857 (2022).
Wiederhold, N. P. et al. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive Candidiasis. Antimicrob. Agents Chemother. 65, e02694–02620 (2021).
Larkin, E. et al. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.02396-16 (2017).
Marcos-Zambrano, L. J., Gomez-Perosanz, M., Escribano, P., Bouza, E. & Guinea, J. The novel oral glucan synthase inhibitor SCY-078 shows in vitro activity against sessile and planktonic Candida spp. J. Antimicrob. Chemother. 72, 1969–1976 (2017).
He, R., Lin, F., Yu, B. & Huang, L. Efficacy and safety of ibrexafungerp in the treatment of vulvovaginal candidiasis: a meta-analysis of randomized controlled trials. Heliyon 10, e28776 (2024).
Locke, J. B. et al. Outcomes by Candida spp. in the ReSTORE phase 3 trial of rezafungin versus caspofungin for candidemia and/or invasive candidiasis. Antimicrob. Agents Chemother. 68, e0158423 (2024).
Thompson, G. R. 3rd et al. Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: pooled data from two prospective randomised controlled trials. Lancet Infect. Dis. 24, 319–328 (2024).
Chandra, J. & Ghannoum, M. A. CD101, a novel echinocandin, possesses potent antibiofilm activity against early and mature Candida albicans biofilms. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.01750-17 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05421858 (2024).
Shaw, K. J. & Ibrahim, A. S. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J. Fungi. 6, 239 (2020).
Watanabe, N. A. et al. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob. Agents Chemother. 56, 960–971 (2012).
Kriegl, L., Egger, M., Boyer, J., Hoenigl, M. & Krause, R. New treatment options for critically important WHO fungal priority pathogens. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2024.03.006 (2024).
Borba-Santos, L. P. et al. Screening of pandemic response box library reveals the high activity of olorofim against pathogenic sporothrix species. J. Fungi. https://doi.org/10.3390/jof8101004 (2022).
Kirchhoff, L. et al. Inhibition of azole-resistant Aspergillus fumigatus biofilm at various formation stages by antifungal drugs, including olorofim. J. Antimicrob. Chemother. 77, 1645–1654 (2022).
Wall, G., Chen, E., Hull, M. V. & Lopez-Ribot, J. L. Screening the CALIBR ReFRAME library in search for inhibitors of Candida auris biofilm formation. Front. Cell Infect. Microbiol. 10, 597931 (2020).
Wall, G. & Lopez-Ribot, J. L. Screening repurposing libraries for identification of drugs with novel antifungal activity. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.00924-20 (2020).
Ajetunmobi, O. H. et al. High-throughput screening of the repurposing hub library to identify drugs with novel inhibitory activity against Candida albicans and Candida auris biofilms. J. Fungi. https://doi.org/10.3390/jof9090879 (2023).
Zhao, M. et al. Turbinmicin inhibits Candida biofilm growth by disrupting fungal vesicle-mediated trafficking. J. Clin. Invest. https://doi.org/10.1172/JCI145123 (2021). This study identifies turbinmicin as a novel extracellular vesicle-targeting antifungal.
Guha, S. et al. Optimization of the antifungal properties of the bacterial peptide EntV by variant analysis. mBio 15, e0057024 (2024).
AlJindan, R. & AlEraky, D. M. Silver nanoparticles: a promising antifungal agent against the growth and biofilm formation of the emergent Candida auris. J. Fungi. https://doi.org/10.3390/jof8070744 (2022).
Miyazima, T. Y., Ishikawa, K. H., Mayer, M. P. A., Saad, S. M. I. & Nakamae, A. E. M. Cheese supplemented with probiotics reduced the Candida levels in denture wearers—RCT. Oral. Dis. 23, 919–925 (2017).
Chakrabarti, A. et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 119, 1809–1818 (2009).
Mello, T. P., Lackner, M., Branquinha, M. H. & Santos, A. L. S. Impact of biofilm formation and azoles’ susceptibility in Scedosporium/Lomentospora species using an in vitro model that mimics the cystic fibrosis patients’ airway environment. J. Cyst. Fibros. 20, 303–309 (2021).
Parize, P. et al. Outcome of patients with cystic fibrosis colonized by Scedosporium and Lomentospora species: a longitudinal cohort study. Med. Mycol. https://doi.org/10.1093/mmy/myad051 (2023).
Grimshaw, S. G. et al. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS ONE 14, e0225796 (2019).
Ghannoum, M. & Isham, N. Fungal nail infections (onychomycosis): a never-ending story? PLoS Pathog. 10, e1004105 (2014).
Pinto, H., Simoes, M. & Borges, A. Prevalence and impact of biofilms on bloodstream and urinary tract infections: a systematic review and meta-analysis. Antibiotics https://doi.org/10.3390/antibiotics10070825 (2021).
Miesel, L., Cushion Melanie, T., Ashbaugh, A., Lopez Santiago, R. & Ong, V. Efficacy of rezafungin in prophylactic mouse models of invasive candidiasis, aspergillosis, and pneumocystis pneumonia. Antimicrob. Agents Chemother. 65, e01992-20 (2021).
Goje, O. et al. Oral ibrexafungerp for vulvovaginal candidiasis treatment: an analysis of VANISH 303 and VANISH 306. J. Women’s Health 32, 178–186 (2023).
Kirchhoff, L. et al. Antibiofilm activity of antifungal drugs, including the novel drug olorofim, against Lomentospora prolificans. J. Antimicrob. Chemother. 75, 2133–2140 (2020).
Gintjee, T. J., Donnelley, M. A. & Thompson, G. R., III. Aspiring antifungals: review of current antifungal pipeline developments. J. Fungi. https://doi.org/10.3390/jof6010028 (2020).
Kean, R. & Ramage, G. Combined antifungal resistance and biofilm tolerance: the global threat of Candida auris. mSphere https://doi.org/10.1128/mSphere.00458-19 (2019).
Martinez, L. R. & Casadevall, A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 73, 4592–4601 (2007).
Williams, C., Rajendran, R. & Ramage, G. Aspergillus biofilms in human disease. Adv. Exp. Med. Biol. 931, 1–11 (2016).
Hoyer, L. L. & Cota, E. Candida albicans agglutinin-like sequence (Als) family vignettes: a review of Als protein structure and function. Front. Microbiol. 7, 280 (2016).
Ramage, G., VandeWalle, K., Lopez-Ribot, J. L. & Wickes, B. L. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214, 95–100 (2002).
Dominguez, E. et al. Conservation and divergence in the Candida species biofilm matrix mannan–glucan complex structure, function, and genetic control. mBio https://doi.org/10.1128/mBio.00451-18 (2018).
Nobile, C. J. et al. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 7, e1000133 (2009).
Dominguez, E. G. et al. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere https://doi.org/10.1128/mSphereDirect.00680-18 (2019).
Bottcher, B. et al. A highly conserved tRNA modification contributes to C. albicans filamentation and virulence. Microbiol. Spectr. 12, e0425522 (2024).
Gravelat, F. N. et al. Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol. 12, 473–488 (2010).
Rajendran, R. et al. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 55, 2092–2097 (2011).
Wang, L., Tian, X., Gyawali, R. & Lin, X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc. Natl Acad. Sci. USA 110, 11571–11576 (2013).
Ramage, G. Comparing apples and oranges: considerations for quantifying candidal biofilms with XTT [2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide] and the need for standardized testing. J. Med. Microbiol. 65, 259–260 (2016).
Acknowledgements
The authors acknowledge M. Butcher and H. Abduljalil (Glasgow Caledonian University) for their helpful comments and feedback during the development of the figures within this Review.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
G.R. has received speaker fees from Gilead and Mundipharma. R.R.-R. has received speaker fees from Mundipharma, Astellas, Basilea, Gilead, Pfizer and Scynexis, and is the principal investigator for phase II clinical trials for Scynexis and F2G. The other authors do not declare any conflict of interest.
Peer review
Peer review information
Nature Reviews Microbiology thanks Yue Qu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramage, G., Kean, R., Rautemaa-Richardson, R. et al. Fungal biofilms in human health and disease. Nat Rev Microbiol 23, 355–370 (2025). https://doi.org/10.1038/s41579-025-01147-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-025-01147-0
This article is cited by
-
Clinical profiling, antifungal drug susceptibility, and biofilm formation ability in pulmonary mucormycosis
BMC Microbiology (2025)
-
Optimized electroporation for efficient evaluation of genetic elements in Dichomitus squalens
World Journal of Microbiology and Biotechnology (2025)