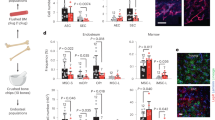
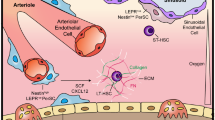
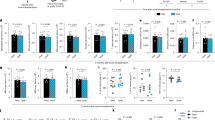
Abstract
Blood production depends on rare haematopoietic stem cells (HSCs) and haematopoietic stem and progenitor cells (HSPCs) that ultimately take up residence in the bone marrow during development. HSPCs and HSCs are subject to extrinsic regulation by the bone marrow microenvironment, or niche. Studying the interactions between HSCs and their niche is critical for improving ex vivo culturing conditions and genetic manipulation of HSCs, which is pivotal for improving autologous HSC therapies and transplantations. Additionally, understanding how the complex molecular network in the bone marrow is altered during ageing is paramount for developing novel therapeutics for ageing-related haematopoietic disorders. HSCs are unique amongst stem and progenitor cell pools in that they engage with multiple physically distinct niches during their ontogeny. HSCs are specified from haemogenic endothelium in the aorta, migrate to the fetal liver and, ultimately, colonize their final niche in the bone marrow. Recent studies employing single-cell transcriptomics and microscopy have identified novel cellular interactions that govern HSC specification and engagement with their niches throughout ontogeny. New lineage-tracing models and microscopy tools have raised questions about the numbers of HSCs specified, as well as the functional consequences of HSCs interacting with each developmental niche. Advances have also been made in understanding how these niches are modified and perturbed during ageing, and the role of these altered interactions in haematopoietic diseases. In this Review, we discuss these new findings and highlight the questions that remain to be explored.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
26 September 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41580-024-00787-z
References
Osawa, M., Hanada, K.-I., Hamada, H. & Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245 (1996).
Calvanese, V. & Mikkola, H. K. The genesis of human hematopoietic stem cells. Blood 142, 519–532 (2023).
Bertrand, J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010).
Boisset, J.-C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010).
Ema, H. & Nakauchi, H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95, 2284–2288 (2000).
Morrison, S. J., Hemmati, H. D., Wandycz, A. M. & Weissman, I. L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl Acad. Sci. USA 92, 10302–10306 (1995).
Murayama, E. et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975 (2006).
White, R. M. et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 (2008).
Sugden, W. W. & North, T. E. Making blood from the vessel: extrinsic and environmental cues guiding the endothelial-to-hematopoietic transition. Life 11, 1027 (2021).
Slukvin, I. I. & Uenishi, G. I. Arterial identity of hemogenic endothelium: a key to unlock definitive hematopoietic commitment in human pluripotent stem cell cultures. Exp. Hematol. 71, 3–12 (2019).
Medvinsky, A. & Dzierzak, E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906 (1996).
Müller, A. M., Medvinsky, A., Strouboulis, J., Grosveld, F. & Dzierzakt, E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301 (1994).
Ivanovs, A. et al. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta–gonad–mesonephros region. J. Exp. Med. 208, 2417–2427 (2011).
Tamplin, O. J. et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252 (2015).
North, T. E. et al. Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736–748 (2009).
North, T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007).
Ganuza, M., Clements, W. & McKinney-Freeman, S. Specification of hematopoietic stem cells in mammalian embryos: a rare or frequent event? Blood 140, 309–320 (2022).
Hou, S. et al. Embryonic endothelial evolution towards first hematopoietic stem cells revealed by single-cell transcriptomic and functional analyses. Cell Res. 30, 376–392 (2020).
Jaffredo, T., Gautier, R., Eichmann, A. & Dieterlen-Lièvre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125, 4575–4583 (1998).
Sugiyama, D. et al. Erythropoiesis from acetyl LDL incorporating endothelial cells at the preliver stage. Blood 101, 4733–4738 (2003).
Oberlin, E., El Hafny, B., Petit-Cocault, L. & Souyri, M. Definitive human and mouse hematopoiesis originates from the embryonic endothelium: a new class of HSCs based on VE-cadherin expression. Int. J. Dev. Biol. 54, 1165–1173 (2010).
Zovein, A. C. et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625–636 (2008).
Ottersbach, K. Endothelial-to-haematopoietic transition: an update on the process of making blood. Biochem. Soc. Trans. 47, 591–601 (2019).
Bloom, W. & Bartelmez, G. Hematopoiesis in young human embryos. Am. J. Anat. 67, 21–53 (1940). This historical paper identifies the emergence of haematopoietic cells in human embryos.
Ohneda, O. et al. Hematopoietic stem cell maintenance and differentiation are supported by embryonic aorta–gonad–mesonephros region-derived endothelium. Blood 92, 908–919 (1998).
Xu, M.-J. et al. Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta–gonad–mesonephros-derived stromal cell lines. Blood 92, 2032–2040 (1998).
Matsuoka, S. et al. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta–gonad–mesonephros region-derived stromal cells. Blood 98, 6–12 (2001).
Oostendorp, R. A. et al. Stromal cell lines from mouse aorta–gonads–mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood 99, 1183–1189 (2002).
McGarvey, A. C. et al. A molecular roadmap of the AGM region reveals BMPER as a novel regulator of HSC maturation. J. Exp. Med. 214, 3731–3751 (2017).
Crosse, E. I. et al. Multi-layered spatial transcriptomics identify secretory factors promoting human hematopoietic stem cell development. Cell Stem Cell 27, 822–839.e8 (2020).
Richard, C. et al. Endothelio-mesenchymal interaction controls runx1 expression and modulates the notch pathway to initiate aortic hematopoiesis. Dev. Cell 24, 600–611 (2013).
Clements, W. K. et al. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474, 220–224 (2011).
Gama-Norton, L. et al. Notch signal strength controls cell fate in the haemogenic endothelium. Nat. Commun. 6, 8510 (2015).
Robert-Moreno, À., Espinosa, L., de la Pompa, J. L. & Bigas, A. RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132, 1117–1126 (2005).
Nottingham, W. T. et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood 110, 4188–4197 (2007).
Kumano, K. et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18, 699–711 (2003).
Hadland, B. K. et al. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 104, 3097–3105 (2004).
North, T. et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575 (1999).
Kissa, K. & Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010).
Thambyrajah, R. et al. cis inhibition of NOTCH1 through JAGGED1 sustains embryonic hematopoietic stem cell fate. Nat. Commun. 15, 1604 (2024). This work identifies cis-acting NOTCH signalling in pre-haemogenic endothelium that regulates haemogenic endothelium transcriptional programmes and specification.
Fitch, S. R. et al. Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell 11, 554–566 (2012).
Damm, E. W. & Clements, W. K. Pdgf signalling guides neural crest contribution to the haematopoietic stem cell specification niche. Nat. Cell Biol. 19, 457–467 (2017).
Chandrakanthan, V. et al. Mesoderm-derived PDGFRA+ cells regulate the emergence of hematopoietic stem cells in the dorsal aorta. Nat. Cell Biol. 24, 1211–1225 (2022).
da Bandeira, D. S. et al. PDGFRβ+ cells play a dual role as hematopoietic precursors and niche cells during mouse ontogeny. Cell Rep. 40, 111114 (2022).
Lan, W. et al. A subset of megakaryocytes regulates development of hematopoietic stem cell precursors. EMBO J. 43, 1722–1739 (2024).
Espín-Palazón, R. et al. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159, 1070–1085 (2014).
Kumaravelu, P. et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta–gonad–mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 129, 4891–4899 (2002).
de Bruijn, M. F., Speck, N. A., Peeters, M. C. & Dzierzak, E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465–2474 (2000).
Eich, C. et al. In vivo single cell analysis reveals Gata2 dynamics in cells transitioning to hematopoietic fate. J. Exp. Med. 215, 233–248 (2018).
Boisset, J.-C. et al. Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood 125, 465–469 (2015).
Calvanese, V. et al. Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature 604, 534–540 (2022). This work presents a single-cell transcriptional map that identifies a set of six transcription factors that define human haematopoietic stem cells through ontogeny.
Ganuza, M. et al. Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny. Nat. Cell Biol. 19, 1153–1163 (2017). This study of sample-to-sample variance of a multicoloured lineage-tracing system identifies a larger pool of specified HSPCs than previously established in mice.
Ganuza, M. et al. Murine foetal liver supports limited detectable expansion of life-long haematopoietic progenitors. Nat. Cell Biol. 24, 1475–1486 (2022). In this work, lineage-tracing models reveal that whereas the fetal liver HSC pool is actively proliferating, many fetal liver HSCs are biased to differentiation and thus, contrary to current models, there is expansion of only about twofold of fetal liver HSCs.
Taoudi, S. et al. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell 3, 99–108 (2008).
Zeng, Y. et al. Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. Cell Res. 29, 881–894 (2019).
Zhou, F. et al. Tracing haematopoietic stem cell formation at single-cell resolution. Nature 533, 487–492 (2016).
Chen, M. J. et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9, 541–552 (2011).
Dignum, T. et al. Multipotent progenitors and hematopoietic stem cells arise independently from hemogenic endothelium in the mouse embryo. Cell Rep. 36, 109675 (2021).
Ganuza, M., Hall, T., Obeng, E. A. & McKinney-Freeman, S. Clones assemble! The clonal complexity of blood during ontogeny and disease. Exp. Hematol. 83, 35–47 (2020).
Kobayashi, M. et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc. Natl Acad. Sci. USA 111, 12151–12156 (2014).
Mikkola, H. K. & Orkin, S. H. The journey of developing hematopoietic stem cells. Development 133, 3733–3744 (2006).
Ivanovs, A., Rybtsov, S., Anderson, R. A. & Medvinsky, A. Vast self-renewal potential of human AGM region HSCs dramatically declines in the umbilical cord blood. Stem Cell Rep. 15, 811–816 (2020).
Van Handel, B. et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood 116, 3321–3330 (2010).
Robin, C. et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell 5, 385–395 (2009).
Gekas, C., Dieterlen-Lièvre, F., Orkin, S. H. & Mikkola, H. K. The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365–375 (2005).
Christensen, J. L., Wright, D. E., Wagers, A. J. & Weissman, I. L. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2, e75 (2004).
Wolber, F. M. et al. Roles of spleen and liver in development of the murine hematopoietic system. Exp. Hematol. 30, 1010–1019 (2002).
Inra, C. N. et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527, 466–471 (2015).
Theodore, L. N. et al. Distinct roles for matrix metalloproteinases 2 and 9 in embryonic hematopoietic stem cell emergence, migration, and niche colonization. Stem Cell Rep. 8, 1226–1241 (2017).
Mazo, I. B., Massberg, S. & von Andrian, U. H. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 32, 493–503 (2011).
Hirsch, E., Iglesias, A., Potocnik, A. J., Hartmann, U. & Fässler, R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature 380, 171–175 (1996).
Potocnik, A. J., Brakebusch, C. & Fässler, R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 12, 653–663 (2000).
Peixoto, M. M. et al. Spatiotemporal dynamics of cytokines expression dictate fetal liver hematopoiesis. Preprint at bioRxiv https://doi.org/10.1101/2023.08.24.554612 (2023).
Helbling, P. M. et al. Tissue-scale dynamic mapping of hematopoietic stem cells and supportive niche cells in the fetal liver. Preprint at bioRxiv https://doi.org/10.1101/2023.09.12.554625 (2023).
Chou, S. & Lodish, H. F. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl Acad. Sci. USA 107, 7799–7804 (2010).
Lee, Y., Leslie, J., Yang, Y. & Ding, L. Hepatic stellate and endothelial cells maintain hematopoietic stem cells in the developing liver. J. Exp. Med. 218, e20200882 (2020).
Rybtsov, S. et al. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43−embryonic precursor. Stem Cell Rep. 3, 489–501 (2014). This work shows that HSCs move through multiple discrete, stepwise developmental stages in the AGM, characterized by specific phenotypic markers and HSC transcriptional regulators.
Ganuza, M. et al. Murine hematopoietic stem cell activity is derived from pre-circulation embryos but not yolk sacs. Nat. Commun. 9, 5405 (2018).
Bowie, M. B. et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest. 116, 2808–2816 (2006).
Bowie, M. B. et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl Acad. Sci. USA 104, 5878–5882 (2007).
McGrath, K. E. et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 11, 1892–1904 (2015).
Fantin, A. et al. KIT is required for fetal liver hematopoiesis. Front. Cell Dev. Biol. 9, 648630 (2021).
Arora, N. et al. Effect of developmental stage of HSC and recipient on transplant outcomes. Dev. Cell 29, 621–628 (2014).
Rybtsov, S., Ivanovs, A., Zhao, S. & Medvinsky, A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 143, 1284–1289 (2016).
Popescu, D.-M. et al. Decoding human fetal liver haematopoiesis. Nature 574, 365–371 (2019).This work presents single-cell sequencing and identification of human fetal liver haematopoietic microenvironment populations and modelling of haematopoietic differentiation trajectories.
Mahony, C. B. & Bertrand, J. Y. How HSCs colonize and expand in the fetal niche of the vertebrate embryo: an evolutionary perspective. Front. Cell Dev. Biol. 7, 34 (2019).
Lewis, K., Yoshimoto, M. & Takebe, T. Fetal liver hematopoiesis: from development to delivery. Stem Cell Res. Ther. 12, 1–8 (2021).
Khan, J. A. et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351, 176–180 (2016). This article shows that fetal liver HSCs engage with portal vessel endothelial cells and Nestin+NG2+ pericytes, creating ‘pocket niches’ that promote HSC proliferation, and that remodelling of these niches later in development may help encourage HSCs to migrate out of the fetal liver.
Zhang, C. C. et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12, 240–245 (2006).
Moore, K. A., Ema, H. & Lemischka, I. R. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood 89, 4337–4347 (1997).
Gao, S. et al. Identification of HSC/MPP expansion units in fetal liver by single-cell spatiotemporal transcriptomics. Cell Res. 32, 38–53 (2022).
Zhao, Y. et al. ATF4 plays a pivotal role in the development of functional hematopoietic stem cells in mouse fetal liver. Blood 126, 2383–2391 (2015).
Zhang, C. C. & Lodish, H. F. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 103, 2513–2521 (2004).
Sugiyama, D., Kulkeaw, K., Mizuochi, C., Horio, Y. & Okayama, S. Hepatoblasts comprise a niche for fetal liver erythropoiesis through cytokine production. Biochem. Biophys. Res. Commun. 410, 301–306 (2011).
Yong, K. S. M. et al. Human CD34loCD133lo fetal liver cells support the expansion of human CD34hiCD133hi hematopoietic stem cells. Cell. Mol. Immunol. 13, 605–614 (2016).
Neo, W. H. et al. Cell-extrinsic hematopoietic impact of Ezh2 inactivation in fetal liver endothelial cells. Blood 131, 2223–2234 (2018).
Shao, L. et al. Hematopoietic Jagged1 is a fetal liver niche factor required for functional maturation and engraftment of fetal hematopoietic stem cells. Proc. Natl Acad. Sci. USA 120, e2210058120 (2023).
Cacialli, P. et al. Synergistic prostaglandin E synthesis by myeloid and endothelial cells promotes fetal hematopoietic stem cell expansion in vertebrates. EMBO J. 41, e108536 (2022).
Sigurdsson, V. et al. Bile acids protect expanding hematopoietic stem cells from unfolded protein stress in fetal liver. Cell Stem Cell 18, 522–532 (2016).
Li, Y. et al. Single-cell analysis of neonatal HSC ontogeny reveals gradual and uncoordinated transcriptional reprogramming that begins before birth. Cell Stem Cell 27, 732–747.e7 (2020).
Hernández-Malmierca, P. et al. Antigen presentation safeguards the integrity of the hematopoietic stem cell pool. Cell Stem Cell 29, 760–775.e10 (2022).
Kieusseian, A., de La Grange, P. B., Burlen-Defranoux, O., Godin, I. & Cumano, A. Immature hematopoietic stem cells undergo maturation in the fetal liver. Development 139, 3521–3530 (2012).
Crisan, M. & Dzierzak, E. The many faces of hematopoietic stem cell heterogeneity. Development 143, 4571–4581 (2016).
Coşkun, S. et al. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 9, 581–590 (2014).
Hall, T. D. et al. Murine fetal bone marrow does not support functional hematopoietic stem and progenitor cells until birth. Nat. Commun. 13, 5403 (2022). This work shows that phenotypically defined fetal bone marrow HSPCs display no repopulating functional activity until near birth, making them functionally and transcriptionally distinct from both fetal liver HSPCs and adult HSPCs.
Charbord, P., Tavian, M., Humeau, L. & Peault, B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood 87, 4109–4119 (1996).
Zheng, Z. et al. Uncovering the emergence of HSCs in the human fetal bone marrow by single-cell RNA-seq analysis. Cell Stem Cell 29, 1562–1579.e7 (2022).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Liu, Y. et al. A specialized bone marrow microenvironment for fetal haematopoiesis. Nat. Commun. 13, 1327 (2022).
Nagasawa, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996).
Zou, Y.-R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Ara, T. et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267 (2003).
Mazo, I. B., Quackenbush, E. J., Lowe, J. B. & von Andrian, U. H. Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow. Blood 99, 4182–4191 (2002).
Kawabata, K. et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc. Natl Acad. Sci. USA 96, 5663–5667 (1999).
Frenette, P. S., Subbarao, S., Mazo, I. B., Von Andrian, U. H. & Wagner, D. D. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc. Natl Acad. Sci. USA 95, 14423–14428 (1998).
Ciriza, J. & García-Ojeda, M. E. Expression of migration-related genes is progressively upregulated in murine Lineage–Sca-1+c-Kit+ population from the fetal to adult stages of development. Stem Cell Res. Ther. 1, 1–13 (2010).
Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006).
Mesnieres, M. et al. Fetal hematopoietic stem cell homing is controlled by VEGF regulating the integrity and oxidative status of the stromal–vascular bone marrow niches. Cell Rep. 36, 109618 (2021).
Galea, G. L., Zein, M. R., Allen, S. & Francis‐West, P. Making and shaping endochondral and intramembranous bones. Dev. Dyn. 250, 414–449 (2021).
Mackie, E. et al. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J. Endocrinol. 40, 46–62 (2008).
Blumer, M. J. Bone tissue and histological and molecular events during development of the long bones. Ann. Anat. / Anatomischer Anz. 235, 151704 (2021).
Maes, C. Role and regulation of vascularization processes in endochondral bones. Calcif. Tissue Int. 92, 307–323 (2013).
Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010).
Sivan, U., De Angelis, J. & Kusumbe, A. P. Role of angiocrine signals in bone development, homeostasis and disease. Open. Biol. 9, 190144 (2019).
Chan, C. K. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009).
Jardine, L. et al. Blood and immune development in human fetal bone marrow and Down syndrome. Nature 598, 327–331 (2021).
Omatsu, Y., Seike, M., Sugiyama, T., Kume, T. & Nagasawa, T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 508, 536–540 (2014).
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20, 303–320 (2019).
Kara, N. et al. Endothelial and Leptin receptor+ cells promote the maintenance of stem cells and hematopoiesis in early postnatal murine bone marrow. Dev. Cell 58, 348–360.e6 (2023).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Shu, H. S. et al. Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell 28, 2122–2136.e3 (2021).
Pineault, K. M., Song, J. Y., Kozloff, K. M., Lucas, D. & Wellik, D. M. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat. Commun. 10, 3168 (2019).
Copley, M. R. et al. The Lin28b–let-7–Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat. Cell Biol. 15, 916–925 (2013).
Li, Y. et al. Basal type I interferon signaling has only modest effects on neonatal and juvenile hematopoiesis. Blood Adv. 7, 2609–2621 (2023).
Wang, C. et al. Lineage-selective super enhancers mediate core regulatory circuitry during adipogenic and osteogenic differentiation of human mesenchymal stem cells. Cell Death Dis. 13, 866 (2022).
Kasbekar, M., Mitchell, C. A., Proven, M. A. & Passegué, E. Hematopoietic stem cells through the ages: a lifetime of adaptation to organismal demands. Cell Stem Cell 30, 1403–1420 (2023).
Mansell, E., Lin, D. S., Loughran, S. J., Milsom, M. D. & Trowbridge, J. J. New insight into the causes, consequences, and correction of hematopoietic stem cell aging. Exp. Hematol. 125–126, 1–5 (2023).
Flach, J. et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512, 198–202 (2014).
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007).
Ho, T. T. et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature 543, 205–210 (2017).
Chua, B. A. et al. Hematopoietic stem cells preferentially traffic misfolded proteins to aggresomes and depend on aggrephagy to maintain protein homeostasis. Cell Stem Cell 30, 460–472.e6 (2023).
Mejia-Ramirez, E., Geiger, H. & Florian, M. C. Loss of epigenetic polarity is a hallmark of hematopoietic stem cell aging. Hum. Mol. Genet. 29, R248–R254 (2020).
Nakamura-Ishizu, A., Ito, K. & Suda, T. Hematopoietic stem cell metabolism during development and aging. Dev. Cell 54, 239–255 (2020).
Ho, Y.-H. et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell 25, 407–418.e6 (2019).
Matteini, F., Mulaw, M. A. & Florian, M. C. Aging of the hematopoietic stem cell niche: new tools to answer an old question. Front. Immunol. 12, 738204 (2021).
Comazzetto, S., Shen, B. & Morrison, S. J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 56, 1848–1860 (2021).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228 (2019).
Bandyopadhyay, S. et al. Mapping the cellular biogeography of human bone marrow niches using single-cell transcriptomics and proteomic imaging. Cell 187, 3120–3140.e29 (2024). This study describes human bone marrow, including the niche, in steady-state and leukaemic conditions at single-cell resolution and with spatial information.
Sarachakov, A. et al. Spatial mapping of human hematopoiesis at single-cell resolution reveals aging-associated topographic remodeling. Blood 142, 2282–2295 (2023). This work analyses the spatial anatomy of haematopoiesis in human bone marrow and reveals age-specific alterations of the bone marrow microenvironment.
Guidi, N. et al. Osteopontin attenuates aging‐associated phenotypes of hematopoietic stem cells. EMBO J. 36, 840–853 (2017).
Ergen, A. V., Boles, N. C. & Goodell, M. A. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 119, 2500–2509 (2012).
Young, K. et al. Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell 28, 1473–1482.e7 (2021). This work shows that reduced production of IGF1 by middle-aged bone marrow MSCs contributes to declining HSC function and that IGF1 treatment of middle-aged HSCs rescues ageing hallmarks.
Guidi, N. et al. An aged bone marrow niche restrains rejuvenated hematopoietic stem cells. Stem Cell 39, 1101–1106 (2021).
Montserrat-Vazquez, S. et al. Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice. npj Regen. Med. 7, 78 (2022). This work shows that systemic treatment of aged mice with CDC42 inhibitor (CASIN) improves HSC function and extends the lifespan of treated mice.
Amoah, A. et al. Aging of human hematopoietic stem cells is linked to changes in Cdc42 activity. Haematologica 107, 393 (2022). This article shows that increased activity of CDC42 and subsequent loss of HSC polarity is conserved between mouse and human HSCs, and that CDC42 inhibition with CASIN rejuvenates human HSCs.
Kuribayashi, W. et al. Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche. J. Exp. Med. 218, e20192283 (2020).
Vionnie, W. et al. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell 167, 1310–1322.e17 (2016).
Meng, Y. et al. Epigenetic programming defines haematopoietic stem cell fate restriction. Nat. Cell Biol. 25, 1–11 (2023).
Sun, D. et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 14, 673–688 (2014).
Florian, M. C. et al. Aging alters the epigenetic asymmetry of HSC division. PLoS Biol. 16, e2003389 (2018).
Rodrigues, C. P., Shvedunova, M. & Akhtar, A. Epigenetic regulators as the gatekeepers of hematopoiesis. Trends Genet. 37, 125–142 (2021).
Derecka, M. et al. EBF1-deficient bone marrow stroma elicits persistent changes in HSC potential. Nat. Immunol. 21, 261–273 (2020). This work shows that an impaired bone marrow niche affects chromatin accessibility in HSCs, especially for myeloid lineage regulators, and that some of these chromatin alterations persist in serial transplantation even after re-introduction of HSCs to the wild-type niche.
Itokawa, N. et al. Epigenetic traits inscribed in chromatin accessibility in aged hematopoietic stem cells. Nat. Commun. 13, 2691 (2022). This work shows that altered chromatin accessibility is specific to aged HSCs compared with mature blood cells and is enriched for binding motifs of transcription factors responsive to external stimuli, which is a potential explanation for how HSCs ‘memorize’ stress signals they experienced during life.
Almeida, M. & O’Brien, C. A. Basic biology of skeletal aging: role of stress response pathways. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 68, 1197–1208 (2013).
Helbling, P. M. et al. Global transcriptomic profiling of the bone marrow stromal microenvironment during postnatal development, aging, and inflammation. Cell Rep. 29, 3313–3330.e4 (2019).
Khosla, S. & Riggs, B. L. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol. Metab. Clin. 34, 1015–1030 (2005).
Gurevitch, O., Slavin, S. & Feldman, A. G. Conversion of red bone marrow into yellow—cause and mechanisms. Med. Hypotheses 69, 531–536 (2007).
Moore, S. G. & Dawson, K. L. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology 175, 219–223 (1990).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784.e6 (2017).
Poulos, M. G. et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J. Clin. Invest. 127, 4163–4178 (2017).
Stucker, S., Chen, J., Watt, F. E. & Kusumbe, A. P. Bone angiogenesis and vascular niche remodeling in stress, aging, and diseases. Front. Cell Dev. Biol. 8, 602269 (2020).
Kusumbe, A. P. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384 (2016).
Maryanovich, M. et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24, 782–791 (2018).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Mitchell, C. A. et al. Stromal niche inflammation mediated by IL-1 signalling is a targetable driver of haematopoietic ageing. Nat. Cell Biol. 25, 30–41 (2023).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013). This study of the secretion of CXCL12 from stromal, haematopoietic, osteoblast and endothelial populations reveals that HSCs occupy a perivascular niche, and that early lymphoid progenitors occupy an endosteal niche.
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Zhu, J. et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706–3712 (2007).
Wu, J. Y. et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proc. Natl Acad. Sci. USA 105, 16976–16981 (2008).
Shen, B. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 591, 438–444 (2021).
Renders, S. et al. Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1. Nat. Commun. 12, 608 (2021).
Saçma, M. et al. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat. Cell Biol. 21, 1309–1320 (2019).
Zhang, J. et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature 590, 457–462 (2021).
Wu, Q. et al. Resilient anatomy and local plasticity of naive and stress haematopoiesis. Nature 627, 839–846 (2024). This work shoes that the microanatomy of bone marrow and lineage-specific production sites are maintained in normal and stress haematopoiesis, but the amplitude and direction of the response to stress signals shows heterogeneity across the skeletal system.
Ganguly, P. et al. The analysis of in vivo aging in human bone marrow mesenchymal stromal cells using colony-forming unit-fibroblast assay and the CD45lowCD271+ phenotype. Stem Cells Int. 2019, 5197983 (2019).
Stolzing, A., Jones, E., Mcgonagle, D. & Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 129, 163–173 (2008).
Yuan, N. et al. Young donor hematopoietic stem cells revitalize aged or damaged bone marrow niche by transdifferentiating into functional niche cells. Aging Cell 22, e13889 (2023).
Infante, A. & Rodríguez, C. I. Osteogenesis and aging: lessons from mesenchymal stem cells. Stem Cell Res. Ther. 9, 1–7 (2018).
Fazeli, P. K. et al. Marrow fat and bone—new perspectives. J. Clin. Endocrinol. Metab. 98, 935–945 (2013).
Schwartz, A. V. Marrow fat and bone: review of clinical findings. Front. Endocrinol. 6, 40 (2015).
Meunier, P., Aaron, J., Edouard, C. & Vlgnon, G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: a quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 80, 147–154 (1971).
Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 (2017).
DiMascio, L. et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J. Immunol. 178, 3511–3520 (2007).
Stier, S. et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201, 1781–1791 (2005).
Nilsson, S. K. et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232–1239 (2005).
Florian, M. C. et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 10, 520–530 (2012).
Gao, X. et al. Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat. Cell Biol. 25, 1–12 (2023).
Aaron, N., Costa, S., Rosen, C. J. & Qiang, L. The implications of bone marrow adipose tissue on inflammaging. Front. Endocrinol. 13, 853765 (2022).
Frisch, B. J. et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via interleukin-1B. JCI Insight 4, e124213 (2019).
Pietras, E. M. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood 130, 1693–1698 (2017).
Bogeska, R. et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29, 1273–1284.e8 (2022).
Pietras, E. M. et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 18, 607–618 (2016).
Yamashita, M. & Passegué, E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell 25, 357–372.e7 (2019).
Valletta, S. et al. Micro-environmental sensing by bone marrow stroma identifies IL-6 and TGFβ1 as regulators of hematopoietic ageing. Nat. Commun. 11, 4075 (2020).
Avagyan, S. & Zon, L. I. Clonal hematopoiesis and inflammation—the perpetual cycle. Trends Cell Biol. 33, 695–707 (2023). This work shows that HSPCs carrying clonal haematopoiesis mutations protect themselves from inflammation derived from their mature progeny by upregulation of inflammation suppressors, which confers a competitive advantage to mutant HSPCs.
Cai, Z. et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell 23, 833–849.e5 (2018).
Cook, E. K. et al. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv. 3, 2482–2486 (2019).
Abegunde, S. O., Buckstein, R., Wells, R. A. & Rauh, M. J. An inflammatory environment containing TNFα favors Tet2-mutant clonal hematopoiesis. Exp. Hematol. 59, 60–65 (2018).
SanMiguel, J. M. et al. Distinct tumor necrosis factor-α receptors dictate stem cell fitness versus lineage output in Dnmt3a-mutant clonal hematopoiesis. Cancer Discov. 12, 2763–2773 (2022).
Zioni, N. et al. Inflammatory signals from fatty bone marrow support DNMT3A driven clonal hematopoiesis. Nat. Commun. 14, 2070 (2023).
Caiado, F., Pietras, E. M. & Manz, M. G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 218, e20201541 (2021).
Craver, B. M., El Alaoui, K., Scherber, R. M. & Fleischman, A. G. The critical role of inflammation in the pathogenesis and progression of myeloid malignancies. Cancers 10, 104 (2018).
de Jong, M. M., Chen, L., Raaijmakers, M. H. & Cupedo, T. Bone marrow inflammation in haematological malignancies. Nat. Rev. Immunol. 24, 543–558 (2024).
Chen, L. et al. A single-cell taxonomy predicts inflammatory niche remodeling to drive tissue failure and outcome in human AML. Blood Cancer Discov. 4, 394–417 (2023).
Avagyan, S. et al. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 374, 768–772 (2021).
Azuma, K. et al. Genetic variations of bone marrow mesenchymal stromal cells derived from acute leukemia and myelodysplastic syndrome by targeted deep sequencing. Leuk. Res. 62, 23–28 (2017).
Abbas, S. et al. Coexistence of aberrant hematopoietic and stromal elements in myelodysplastic syndromes. Blood Cells Mol. Dis. 66, 37–46 (2017).
Bandara, W. M. S., Rathnayake, A. I. S., Neththikumara, N. F., Goonasekera, H. W. & Dissanayake, V. H. Comparative analysis of the genetic variants in haematopoietic stem/progenitor and mesenchymal stem cell compartments in de novo myelodysplastic syndromes. Blood Cells Mol. Dis. 88, 102535 (2021).
Lopez-Villar, O. et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q-syndrome. Leukemia 23, 664–672 (2009).
Teofili, L. et al. Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 117, 2700–2707 (2011).
Bacharach, T., Kaushansky, N. & Shlush, L. I. Age-related micro-environmental changes as drivers of clonal hematopoiesis. Curr. Opin. Hematol. 31, 53–57 (2024).
Méndez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 20, 285–298 (2020).
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cell 4, 7–25 (1978).
Mendoza‐Castrejon, J. & Magee, J. A. Layered immunity and layered leukemogenicity: developmentally restricted mechanisms of pediatric leukemia initiation. Immunol. Rev. 315, 197–215 (2023).
Sivaraj, K. K. et al. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep. 36, 109352 (2021).
Luis, T. C. et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 113, 546–554 (2009).
Ruiz-Herguido, C. et al. Hematopoietic stem cell development requires transient Wnt/β-catenin activity. J. Exp. Med. 209, 1457–1468 (2012).
Genthe, J. R. & Clements, W. K. R-spondin 1 is required for specification of hematopoietic stem cells through Wnt16 and Vegfa signaling pathways. Development 144, 590–600 (2017).
Lee, J. B. et al. Notch–HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent stem cells. Blood 122, 1162–1173 (2013).
Gering, M. & Patient, R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev. Cell 8, 389–400 (2005).
Lawson, N. D., Vogel, A. M. & Weinstein, B. M. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell 3, 127–136 (2002).
Nicoli, S., Tobia, C., Gualandi, L., De Sena, G. & Presta, M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood 111, 4965–4972 (2008).
Wilkinson, R. N. et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev. Cell 16, 909–916 (2009).
Li, Y. et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes. Dev. 28, 2597–2612 (2014).
Florian, M. C. et al. Inhibition of Cdc42 activity extends lifespan and decreases circulating inflammatory cytokines in aged female C57BL/6 mice. Aging Cell 19, e13208 (2020).
Florian, M. C. et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503, 392–396 (2013).
Schreck, C. et al. Niche WNT5A regulates the actin cytoskeleton during regeneration of hematopoietic stem cells. J. Exp. Med. 214, 165–181 (2017).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Ross, J. B. et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature 628, 162–170 (2024).
Zhang, X. et al. Harnessing matrix stiffness to engineer a bone marrow niche for hematopoietic stem cell rejuvenation. Cell Stem Cell 30, 378–395.e8 (2023).
Duguid, A., Mattiucci, D. & Ottersbach, K. Infant leukaemia—faithful models, cell of origin and the niche. Dis. Model. Mech. 14, dmm049189 (2021).
Yamaguchi, T., Kawamoto, E., Gaowa, A., Park, E. J. & Shimaoka, M. Remodeling of bone marrow niches and roles of exosomes in leukemia. Int. J. Mol. Sci. 22, 1881 (2021).
Barrett, N. A. et al. Mll-AF4 confers enhanced self-renewal and lymphoid potential during a restricted window in development. Cell Rep. 16, 1039–1054 (2016).
Menendez, P. et al. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J. Exp. Med. 206, 3131–3141 (2009).
Rowe, R. G. et al. The developmental stage of the hematopoietic niche regulates lineage in MLL-rearranged leukemia. J. Exp. Med. 216, 527–538 (2019).
Boucher, A. C., Caldwell, K. J., Crispino, J. D. & Flerlage, J. E. Clinical and biological aspects of myeloid leukemia in Down syndrome. Leukemia 35, 3352–3360 (2021).
Woo, A. J. et al. Developmental differences in IFN signaling affect GATA1s-induced megakaryocyte hyperproliferation. J. Clin. Invest. 123, 3292–3304 (2013).
Klusmann, J.-H. et al. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes. Dev. 24, 1659–1672 (2010).
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease (R01DK116835 and R01DK104028, to S.M.-F.), National Heart, Lung, and Blood Institute (F31HL170678, to T.L.C.), American Lebanese Syrian Associated Charities (to S.M.-F. and M.D.), Leukaemia Research Foundation (to M.D.) and WES Foundation (to M.D.). S.M.-F. is a Scholar of The Leukaemia & Lymphoma Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Sandra Pinho; Maria Carolina Florian, who co-reviewed with Francesca Matteini; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- β-Catenin
-
A key adhesion protein and transcription factor in the Wnt signalling pathway.
- Aorta–gonad–mesonephros
-
(AGM). An embryonic mesodermal region from which haematopoietic stem cells (HSCs) emerge in mammals.
- Adipokines
-
Cytokines produced by adipose tissue that have roles in inflammatory and metabolic signalling.
- A-ZIP/F1 lipoatrophic mice
-
A mouse model that has significantly reduced white and brown adipose tissue.
- Carnegie stage
-
(CS). A classification system of 23 stages developed as a standard timeline of human embryonic development.
- Catecholamine
-
An aromatic amine that serves as a neurotransmitter, such as dopamine and adrenaline.
- CellComm
-
A bioinformatics algorithm that predicts cell-to-cell communications via ligand–receptor interactions based on single-cell RNA sequencing (scRNA-seq) or spatial transcriptomics data.
- CellPhoneDB
-
A bioinformatic algorithm that can predict ligand–receptor cellular interactions based on single-cell RNA sequencing (scRNA-seq) expression of the ligands, receptors and associated signalling pathway genes across annotated cellular populations.
- Endosteal niche
-
A microenvironment on the outer edge of the bone marrow comprising many cell types, including haematopoietic stem cells (HSCs).
- In cis interactions
-
Interactions between ligand and receptor pairs occurring on the same cell.
- Intermediate cell mass
-
A narrow section of the embryonic mesoderm from which haematopoietic stem cells (HSCs) emerge in zebrafish, and ultimately gives rise to the urogenital systems.
- Lycorine
-
A toxic alkaloid found in some plant species.
- Lymphopenia
-
A haematological disorder in which there is a clinical decrease in the number of lymphocytes present in the blood.
- Metalloproteases
-
Enzymes found in the intercellular space that break down extracellular proteins.
- Myeloid lineage bias
-
Occurs when an individual haematopoietic stem cell (HSC) has an increased relative contribution of myeloid lineage progeny compared with lymphoid lineage progeny.
- Neural crest
-
A group of multipotent stem cells that migrate during development and give rise to large groups of tissues, such as connective tissue, skin pigmentation cells and craniofacial bones.
- Platelet-derived growth factor receptor (PDGFR) signalling
-
A regulator of many developmentally relevant processes in a tissue-dependent manner, including cell proliferation and growth.
- Xenotransplantation
-
The transplantation of organs or tissues, such as blood, between two different species.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cain, T.L., Derecka, M. & McKinney-Freeman, S. The role of the haematopoietic stem cell niche in development and ageing. Nat Rev Mol Cell Biol 26, 32–50 (2025). https://doi.org/10.1038/s41580-024-00770-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-024-00770-8